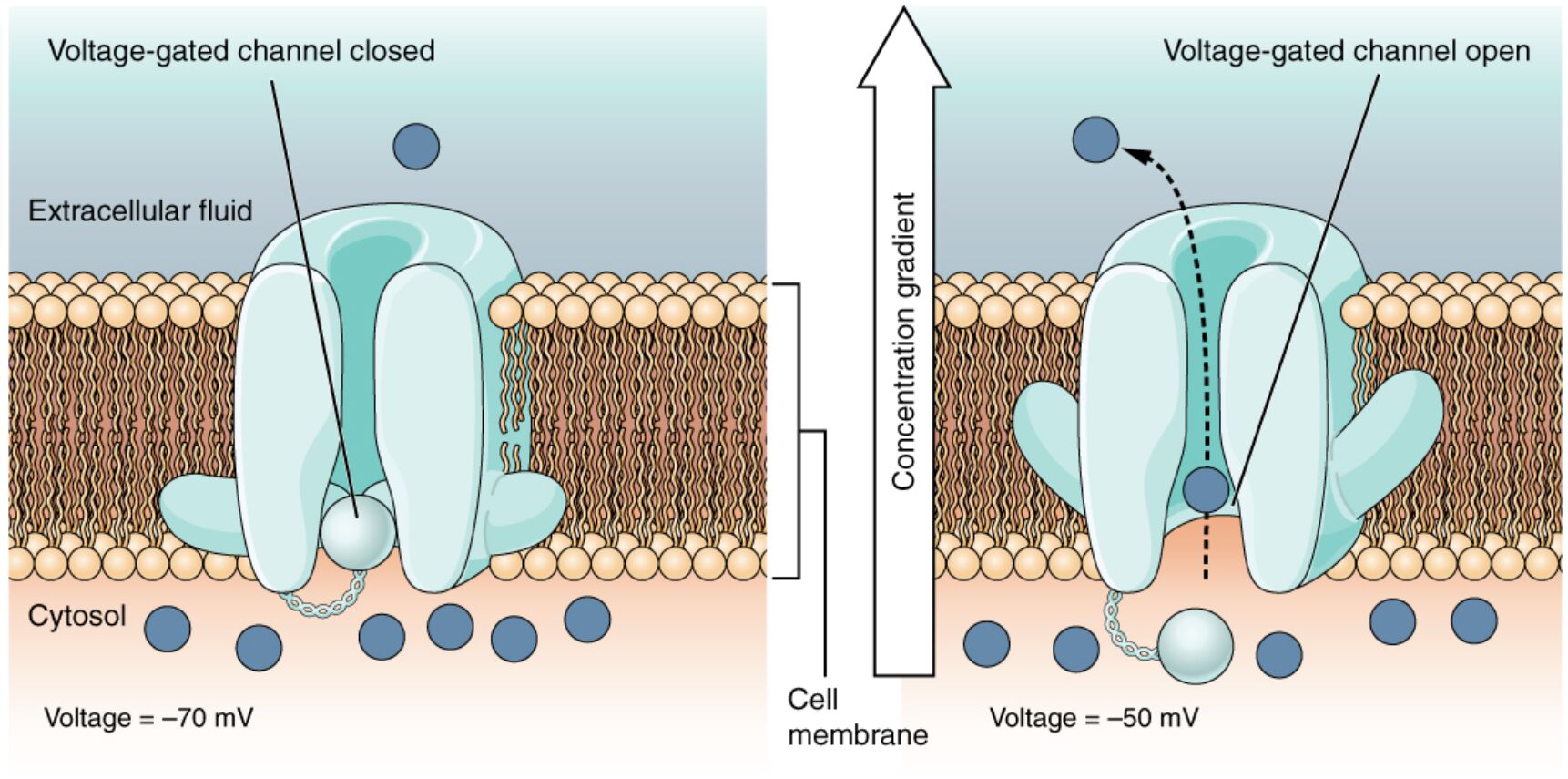
Voltage-gated channels are critical components of cellular membranes, responding to changes in electrical potential to control ion movement across the membrane. This diagram illustrates how these channels open when the transmembrane voltage shifts, with amino acids within the protein structure sensing charge to allow specific ions to pass through. Exploring this mechanism provides key insights into nerve impulse transmission, muscle contraction, and overall cellular communication.
Key Labels in the Voltage-Gated Channels Diagram
This section details each labeled element, offering a clear understanding of how voltage-gated channels function.
Voltage-gated channel: This protein spans the cell membrane, opening in response to changes in transmembrane voltage to allow ion passage. Its activation is essential for generating and propagating electrical signals in excitable cells.
Transmembrane voltage: The electrical potential difference across the cell membrane, which triggers the opening of the channel when it reaches a specific threshold. It reflects the balance of positive and negative charges on either side of the membrane.
Amino acid sensor: These charged amino acids within the channel protein detect changes in transmembrane voltage, initiating a conformational change. Their sensitivity to charge determines the channel’s opening and closing dynamics.
Channel pore: The selective passageway within the voltage-gated channel that opens to allow specific ions, such as sodium or potassium, to cross the membrane. Its structure ensures precise ion selectivity and flow regulation.
Sodium ion (Na+): This positively charged ion moves into the cell when the channel opens, driving depolarization during an action potential. Its influx is critical for initiating nerve and muscle activity.
Potassium ion (K+): This ion exits the cell through the channel during repolarization, helping restore the resting membrane potential. Its movement stabilizes the cell after excitation.
Extracellular space: The area outside the cell where sodium ions are more concentrated, serving as the source for ion influx. It interacts with the channel’s external surface during voltage changes.
Cytoplasm: The internal cell environment where potassium ions are released or sodium ions accumulate, influencing intracellular signaling. It is the destination for ions passing through the channel.
Cell membrane: The lipid bilayer that houses the voltage-gated channel, providing a barrier that maintains the transmembrane voltage. It supports the structural integrity of the channel mechanism.
Mechanism of Voltage-Gated Channels
Voltage-gated channels are pivotal for electrical signaling in excitable tissues. They respond dynamically to voltage shifts.
- The transmembrane voltage changes, detected by the amino acid sensor, open the voltage-gated channel.
- The channel pore allows sodium ion (Na+) influx, initiating depolarization.
- Potassium ion (K+) efflux follows, aiding repolarization.
- The process is rapid, enabling action potential propagation.
- Channels reset to a closed state after voltage normalization.
Role of Transmembrane Voltage in Channel Activation
Transmembrane voltage serves as the primary trigger for channel function. It drives the electrical cycle.
- A shift in transmembrane voltage alters the electric field across the cell membrane.
- The amino acid sensor responds, opening the voltage-gated channel.
- This occurs in neurons and cardiac muscle cells during excitation.
- The threshold varies, typically around -55 mV for sodium channels.
- Voltage changes are influenced by ion gradients and membrane properties.
Ion Movement and Cellular Effects
The flow of sodium ion (Na+) and potassium ion (K+) shapes cellular responses. It underpins excitability.
- Sodium ion (Na+) entry depolarizes the membrane, triggering action potentials.
- Potassium ion (K+) exit repolarizes the membrane, restoring balance.
- This cycle supports nerve impulse transmission and muscle contraction.
- Ion pumps, like the Na+/K+-ATPase, maintain gradient stability.
- Dysregulation can lead to hyperexcitability or paralysis.
Structural and Functional Insights
The cell membrane and voltage-gated channel form a sophisticated system. Their design ensures precision.
- The cell membrane embeds the voltage-gated channel, providing a stable platform.
- Extracellular space and cytoplasm maintain ion concentration gradients.
- The amino acid sensor contains voltage-sensing domains with positive charges.
- Channel subunits assemble to form the channel pore, enhancing selectivity.
- Structural studies inform drug design targeting these channels.
Physiological Significance and Clinical Relevance
Voltage-gated channels have broad physiological roles. Their study guides medical interventions.
- Sodium ion (Na+) channels are targets for local anesthetics like lidocaine.
- Potassium ion (K+) channel dysfunction is linked to arrhythmias.
- Voltage-gated channel abnormalities cause epilepsy and long QT syndrome.
- Neurotoxins, like tetrodotoxin, block these channels, affecting nerve function.
- Research into channel kinetics supports personalized treatments.
In conclusion, the voltage-gated channels diagram illustrates how transmembrane voltage activates the voltage-gated channel via the amino acid sensor, enabling sodium ion (Na+) and potassium ion (K+) movement through the channel pore. This process connects the extracellular space and cytoplasm, driving essential functions like nerve signaling and muscle activity. Understanding these dynamics enhances our grasp of cellular physiology and its therapeutic applications.

