The autonomic control of pupillary size diagram unveils the intricate neural mechanisms that regulate the pupil’s response to light, a vital aspect of visual function and ocular health. This chart illustrates how the sympathetic and parasympathetic systems work in tandem to adjust pupil diameter, responding to environmental light changes via the retina and optic nerve. Delving into this process offers valuable insights into the body’s adaptive responses and the balance maintained by the autonomic nervous system.
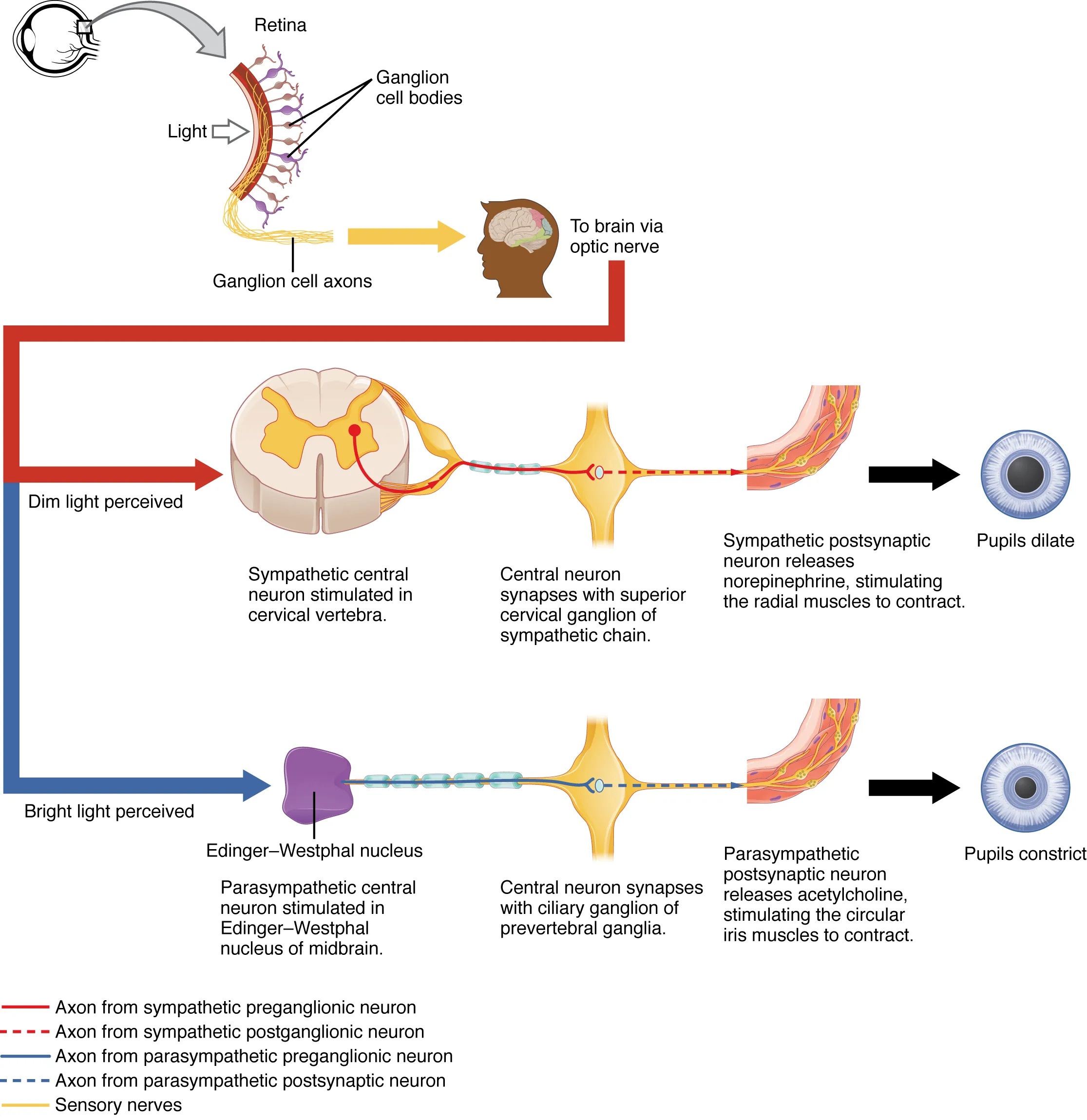
Labeled Components in the Diagram
Retina The retina is the light-sensitive layer at the back of the eye, containing photoreceptors that detect light intensity. It initiates the pupillary reflex by sending signals through ganglion cells to the brain.
Ganglion cell bodies The ganglion cell bodies reside in the retina, processing light input and transmitting signals via their axons. These cells play a critical role in relaying information to the optic nerve for further processing.
Ganglion cell axons The ganglion cell axons extend from the retina, forming the optic nerve that carries visual and light intensity data to the brain. This pathway is essential for coordinating the pupillary light reflex.
To brain via optic nerve The to brain via optic nerve represents the route where ganglion cell axons deliver signals to the brain for interpretation. This connection enables the central nervous system to modulate pupillary responses.
Dim light perceived The dim light perceived indicates a low-light condition detected by the retina, triggering a sympathetic response. This perception leads to pupil dilation to maximize light intake.
Bright light perceived The bright light perceived signifies high-light conditions detected by the retina, initiating a parasympathetic response. This perception results in pupil constriction to protect the inner eye.
Sympathetic central neuron stimulated in cervical vertebra The sympathetic central neuron stimulated in cervical vertebra originates in the spinal cord’s upper thoracic or cervical region. It activates the sympathetic chain to dilate the pupil in dim light.
Central neuron synapses with superior cervical ganglion of sympathetic chain The central neuron synapses with superior cervical ganglion of sympathetic chain involves a preganglionic neuron connecting to the ganglion. This synapse facilitates the release of norepinephrine for pupil dilation.
Sympathetic postsynaptic neuron releases norepinephrine, stimulating the radial muscles to contract The sympathetic postsynaptic neuron releases norepinephrine, stimulating the radial muscles to contract describes the postganglionic neuron’s action. This contraction dilates the pupil to adapt to dim light.
Edinger-Westphal nucleus The Edinger-Westphal nucleus is a parasympathetic nucleus in the midbrain that controls pupil constriction. It sends signals via the oculomotor nerve in response to bright light.
Parasympathetic central neuron stimulated in Edinger-Westphal nucleus of midbrain The parasympathetic central neuron stimulated in Edinger-Westphal nucleus of midbrain initiates the parasympathetic response. This neuron drives pupil constriction to protect the eye from excessive light.
Central neuron synapses with ciliary ganglion of prevertebral ganglia The central neuron synapses with ciliary ganglion of prevertebral ganglia involves a preganglionic neuron connecting to the ciliary ganglion. This synapse supports the parasympathetic control of the iris.
Parasympathetic postsynaptic neuron releases acetylcholine, stimulating the circular iris muscles to contract The parasympathetic postsynaptic neuron releases acetylcholine, stimulating the circular iris muscles to contract details the postganglionic neuron’s role. This contraction constricts the pupil in bright light.
Pupils dilate The pupils dilate is the end result of sympathetic activation, enlarging the pupil to allow more light entry. This response occurs in dim conditions to enhance vision.
Pupils constrict The pupils constrict is the outcome of parasympathetic activation, reducing pupil size to limit light exposure. This occurs in bright conditions to protect the retina.
Axon from sympathetic preganglionic neuron The axon from sympathetic preganglionic neuron extends from the spinal cord to the superior cervical ganglion. It carries the initial sympathetic signal for pupil dilation.
Axon from sympathetic postganglionic neuron The axon from sympathetic postganglionic neuron travels from the ganglion to the iris, releasing norepinephrine. This axon completes the sympathetic pathway for dilation.
Axon from parasympathetic preganglionic neuron The axon from parasympathetic preganglionic neuron runs from the Edinger-Westphal nucleus to the ciliary ganglion. It initiates the parasympathetic response for constriction.
Axon from parasympathetic postsynaptic neuron The axon from parasympathetic postsynaptic neuron extends from the ciliary ganglion to the iris, releasing acetylcholine. This axon finalizes the constriction process.
Sensory nerves The sensory nerves include the optic nerve fibers that transmit light data from the retina to the brain. These nerves are crucial for triggering the pupillary reflex.
Anatomy of the Pupillary Reflex
The pupillary reflex is a dynamic process governed by the autonomic nervous system’s dual control. This reflex adjusts pupil size to optimize vision and protect ocular structures.
- The retina contains rod and cone cells, with ganglion cells integrating light signals.
- Ganglion cell axons form the optic nerve, a cranial nerve II pathway to the brain.
- The Edinger-Westphal nucleus, part of the oculomotor nerve (III), houses parasympathetic preganglionic neurons.
- The superior cervical ganglion serves as the sympathetic relay point in the cervical region.
- Radial muscles in the iris dilate the pupil under sympathetic influence.
- Circular muscles constrict the pupil via parasympathetic activation.
Physiological Mechanisms of Pupil Control
The physiological response to light involves neurotransmitter release and muscle action. This mechanism ensures precise regulation of pupil size based on environmental conditions.
- Dim light activates sympathetic neurons, releasing norepinephrine at the iris.
- Bright light stimulates the Edinger-Westphal nucleus, releasing acetylcholine.
- Sympathetic activation increases cyclic AMP, enhancing radial muscle contraction.
- Parasympathetic stimulation elevates calcium levels, contracting circular muscles.
- The reflex arc includes a feedback loop via the optic nerve to adjust responses.
- Myelinated axons in these pathways ensure rapid signal transmission.
Role of Neurotransmitters
Neurotransmitters are pivotal in mediating pupillary responses. Their specific actions dictate whether the pupil dilates or constricts.
- Norepinephrine binds to alpha-1 receptors on radial muscles for dilation.
- Acetylcholine activates muscarinic receptors on circular muscles for constriction.
- Sympathetic postganglionic neurons synthesize norepinephrine from tyrosine.
- Parasympathetic neurons rely on acetylcholine, produced in cholinergic terminals.
- Enzymatic breakdown by acetylcholinesterase terminates parasympathetic signals.
- These neurotransmitters maintain a balanced autonomic response.
Clinical Relevance
Pupillary responses are critical for diagnosing neurological and ocular conditions. Observing these reflexes provides clues to underlying health issues.
- Unequal pupil sizes (anisocoria) may indicate nerve damage or Horner’s syndrome.
- Fixed dilated pupils can signal brain herniation or severe trauma.
- Constricted pupils might suggest opioid overdose or pontine lesions.
- The swinging flashlight test assesses relative afferent pupillary defect.
- Adie’s tonic pupil reflects parasympathetic denervation.
- Pupillary light reflex testing is standard in coma evaluations.
Advances in Ocular Research
Ongoing studies enhance our understanding of pupillary control and its therapeutic applications. These advancements promise better management of eye-related disorders.
- Optical coherence tomography maps retinal ganglion cell health.
- Neurostimulation techniques target the Edinger-Westphal nucleus.
- Gene therapy explores repairing optic nerve damage.
- Pharmacological agents modulate neurotransmitter activity.
- Artificial intelligence analyzes pupillary responses for diagnostics.
- Wearable devices monitor reflex changes in real-time.
The autonomic control of pupillary size exemplifies the body’s remarkable ability to adapt to its environment through neural precision. Mastering these mechanisms equips professionals to address a range of ocular and neurological challenges, enhancing patient care with each new discovery.

