The somatic efferent pathway represents a critical component of the nervous system responsible for transmitting signals from the central nervous system to skeletal muscles, enabling voluntary movements and precise motor control. This pathway involves specialized neurons that ensure rapid and efficient communication, allowing for actions ranging from simple reflexes to complex coordinated activities. By exploring its structure and function, one gains insight into how the body executes intentional physical responses, highlighting the intricate balance between neural signaling and muscular action.
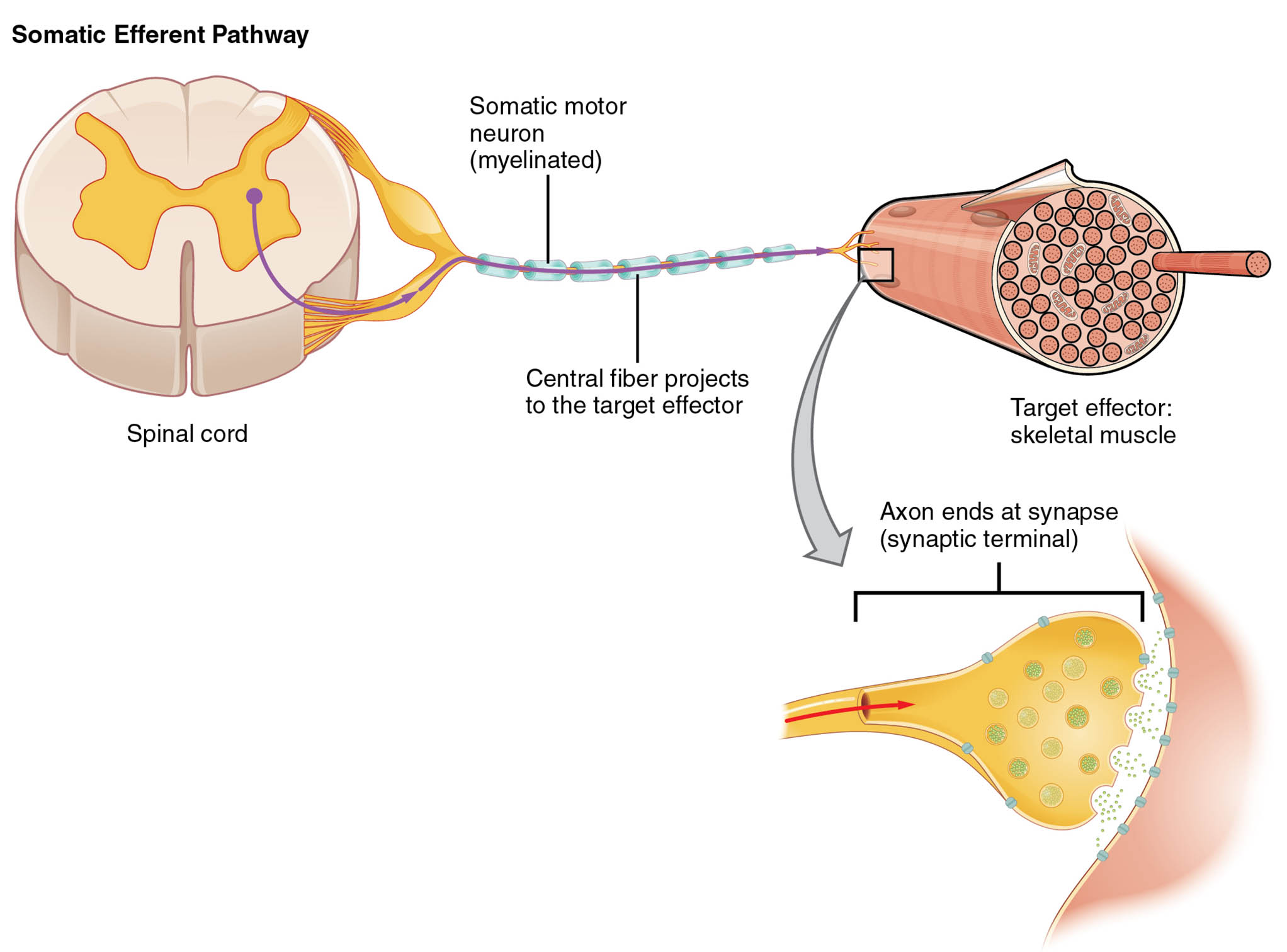
Labeled Components in the Diagram
Spinal cord The spinal cord serves as the origin point for the somatic motor neuron in this pathway, acting as a conduit for signals descending from the brain. It houses the cell bodies of lower motor neurons, which are essential for relaying commands to peripheral effectors.
Somatic motor neuron (myelinated) The somatic motor neuron is a myelinated nerve cell that carries efferent signals from the spinal cord to skeletal muscles. Myelination enhances the speed of action potential propagation, ensuring quick muscle responses during voluntary movements.
Central fiber projects to the target effector The central fiber refers to the axon extension of the somatic motor neuron that projects directly toward the target effector. This projection allows for monosynaptic transmission, minimizing delays in signal delivery to the muscle fibers.
Target effector: skeletal muscle The skeletal muscle acts as the target effector, receiving neural impulses that trigger contraction and movement. It consists of multinucleated fibers capable of generating force through actin-myosin interactions, supporting locomotion and posture.
Axon ends at synapse (synaptic terminal) The axon terminates at a synapse, specifically the synaptic terminal, where neurotransmitters are released to communicate with the muscle cell. This neuromuscular junction facilitates the conversion of electrical signals into chemical ones, leading to muscle depolarization and contraction.
Overview of the Somatic Nervous System
The somatic nervous system encompasses both sensory and motor divisions, with the efferent pathway focusing on motor output to voluntary muscles. Unlike the autonomic system, it operates under conscious control, making it vital for everyday activities.
- This system integrates inputs from higher brain centers, such as the motor cortex, to initiate precise actions.
- Neurons in this pathway are classified as alpha motor neurons, which innervate extrafusal muscle fibers for force generation.
- Myelination by Schwann cells in the peripheral nervous system accelerates conduction velocities up to 120 meters per second.
- Disruptions in this pathway can lead to conditions like paralysis, underscoring its clinical significance.
- Sensory feedback from muscle spindles loops back to refine motor commands, creating a dynamic control system.
Structure and Pathway Details
Beginning at the spinal cord, the somatic efferent pathway follows a direct route to effector organs without intermediate ganglia. This simplicity contrasts with multisynaptic autonomic pathways, allowing for faster responses.
- The pathway originates from ventral horn motor neurons in the spinal cord, whose axons exit via ventral roots.
- These axons form peripheral nerves, traveling through plexuses before reaching target muscles.
- At the neuromuscular junction, the synaptic terminal contains voltage-gated calcium channels that trigger vesicle exocytosis.
- Acetylcholine serves as the primary neurotransmitter, binding to nicotinic receptors on the muscle endplate.
- Post-synaptic potentials here are excitatory, leading to action potentials that propagate along the sarcolemma.
- T-tubules transmit these signals deep into the muscle fiber, initiating calcium release from the sarcoplasmic reticulum.
Physiological Mechanisms of Signal Transmission
Action potentials in somatic motor neurons propagate via saltatory conduction, jumping between nodes of Ranvier for efficiency. This process relies on sodium and potassium ion channels, maintaining membrane potential gradients.
- Depolarization begins at the axon hillock, where graded potentials summate to reach threshold.
- Voltage-gated sodium channels open rapidly, allowing influx that peaks the action potential at +40 mV.
- Repolarization follows with potassium efflux, restoring the resting potential of -70 mV.
- The all-or-none principle ensures consistent signal strength over long distances.
- At the synapse, calcium influx prompts synaptic vesicles to fuse with the presynaptic membrane.
- Released acetylcholine diffuses across the synaptic cleft, a 50 nm gap, to activate ligand-gated channels.
- Enzymatic breakdown by acetylcholinesterase prevents prolonged stimulation, allowing for rapid successive contractions.
Role in Voluntary Movement
Voluntary movements depend on the somatic efferent pathway to translate cortical intentions into muscular actions. Higher centers like the primary motor cortex send descending tracts that synapse with lower motor neurons.
- Corticospinal tracts carry signals for fine motor skills, particularly in distal limbs.
- Vestibulospinal and reticulospinal tracts contribute to balance and postural adjustments.
- Motor units, consisting of one neuron and its innervated fibers, vary in size for graded force control.
- Small motor units activate first for precise tasks, following the size principle.
- Recruitment increases with demand, enabling smooth transitions from weak to strong contractions.
- Fatigue resistance differs among fiber types, with slow-twitch fibers suited for endurance.
Clinical Relevance and Disorders
While the diagram depicts a healthy pathway, understanding its anatomy aids in diagnosing motor dysfunctions. Conditions affecting this system often manifest as weakness or uncoordinated movements.
- Amyotrophic lateral sclerosis targets motor neurons, leading to progressive muscle atrophy.
- Myasthenia gravis impairs neuromuscular transmission through autoantibodies against acetylcholine receptors.
- Spinal cord injuries disrupt efferent signals, causing flaccid paralysis below the lesion site.
- Botulinum toxin inhibits acetylcholine release, used therapeutically for spasticity.
- Electromyography assesses pathway integrity by recording muscle electrical activity.
- Rehabilitation focuses on neuroplasticity to reroute signals around damaged areas.
Comparative Anatomy with Autonomic Pathways
The somatic efferent pathway differs markedly from autonomic ones, featuring a single neuron chain to effectors. Autonomic pathways involve preganglionic and postganglionic neurons for involuntary control.
- Somatic neurons are always excitatory, using acetylcholine exclusively at junctions.
- Autonomic systems employ acetylcholine or norepinephrine, allowing for sympathetic and parasympathetic modulation.
- Myelination in somatic axons supports rapid conduction, essential for reflex arcs.
- Reflexes like the knee-jerk involve both afferent and efferent somatic components.
- Integration with sensory pathways ensures adaptive responses to environmental changes.
Advances in Neurophysiological Research
Recent studies employ optogenetics to manipulate somatic motor neurons, revealing insights into circuit dynamics. Imaging techniques like fMRI map cortical activations linked to efferent outputs.
- Stem cell therapies aim to regenerate damaged motor neurons in spinal injuries.
- Prosthetic interfaces decode efferent signals for controlling artificial limbs.
- Computational models simulate pathway behaviors, predicting outcomes in neurological disorders.
- Pharmacological agents target ion channels to enhance conduction in demyelinating diseases.
- Longitudinal studies track developmental changes in pathway maturation from infancy.
The somatic efferent pathway exemplifies the elegance of neural design, bridging central commands with peripheral execution to facilitate human interaction with the world. Its study not only deepens appreciation for bodily mechanics but also informs therapeutic strategies for motor impairments, promising improved quality of life through ongoing scientific advancements.

