The fibrin synthesis cascade is a critical process in hemostasis, ensuring the formation of blood clots to prevent excessive bleeding. This article delves into the intricate pathways—intrinsic, extrinsic, and the final common pathway—illustrated in the diagram, highlighting the activation of clotting factors. Exploring these mechanisms provides valuable insight into the body’s ability to maintain vascular integrity.
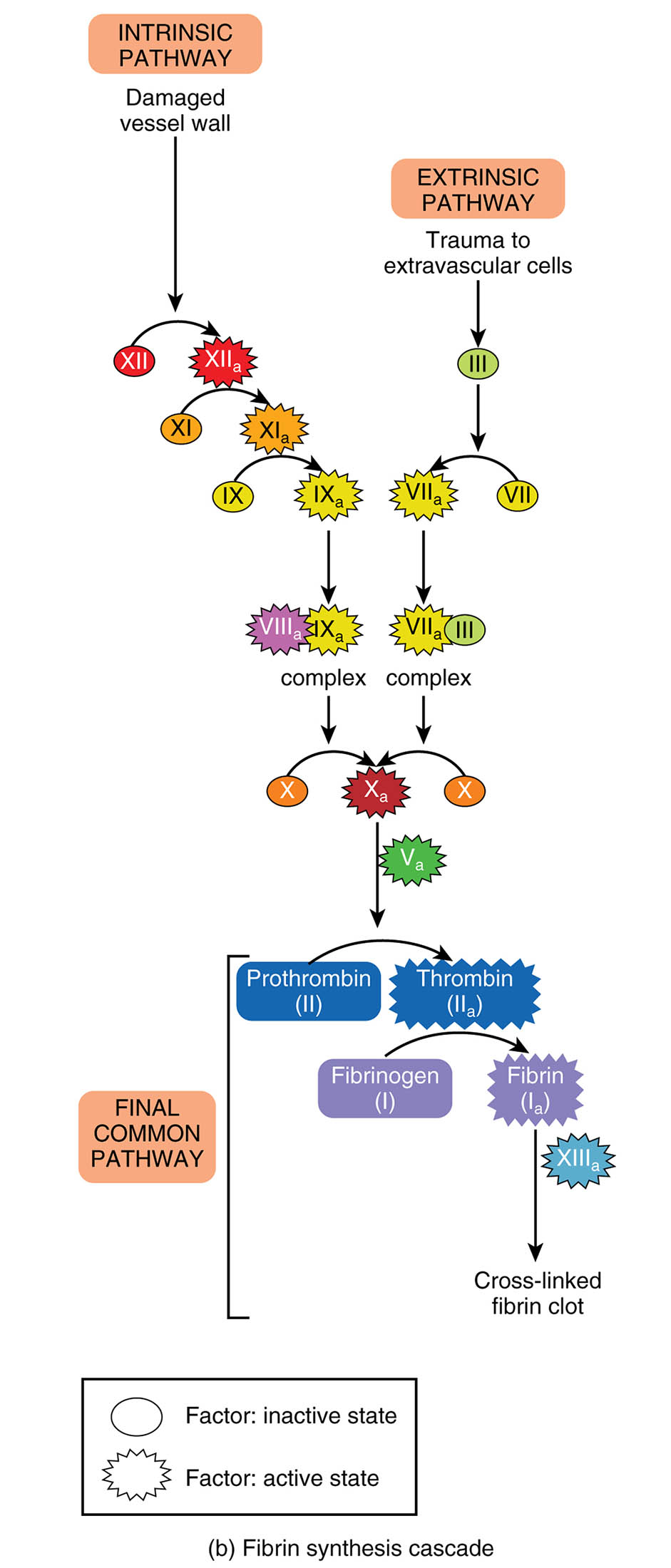
Intrinsic Pathway
- The intrinsic pathway is initiated by damage to the vessel wall, triggering a series of internal clotting factors.
- It involves factors XII, XI, IX, and VIII, culminating in the activation of factor X.
Damaged vessel wall
- The damaged vessel wall exposes subendothelial collagen, initiating the intrinsic pathway of coagulation.
- This exposure activates factor XII, setting off the cascade of clotting reactions.
Extrinsic Pathway
- The extrinsic pathway is triggered by trauma to extravascular cells or tissues, rapidly activating the clotting process.
- It primarily involves factor III (tissue factor) and factor VII, leading to factor X activation.
Trauma to extravascular cells
- Trauma to extravascular cells releases tissue factor, a key initiator of the extrinsic pathway.
- This external injury prompts a swift response to stabilize the injury site.
XII
- Factor XII, in its inactive state, is the first factor activated in the intrinsic pathway upon contact with the damaged vessel wall.
- Once activated to XIIa, it catalyzes the activation of factor XI.
XIIa
- Factor XIIa is the active form of factor XII, playing a pivotal role in amplifying the intrinsic pathway.
- It activates factor XI, advancing the coagulation cascade.
XI
- Factor XI, in its inactive state, is activated by factor XIIa in the intrinsic pathway.
- The activation of XI to XIa contributes to the progression toward factor IX activation.
XIa
- Factor XIa, the active form of factor XI, activates factor IX in the intrinsic pathway.
- This step is crucial for the formation of the tenase complex.
IX
- Factor IX, initially inactive, is activated to IXa by factor XIa in the intrinsic pathway.
- Factor IXa, along with factor VIIIa, forms part of the tenase complex that activates factor X.
IXa
- Factor IXa is the active form of factor IX, essential for the tenase complex in the intrinsic pathway.
- It works with factor VIIIa to convert factor X to its active form, Xa.
VIII
- Factor VIII, in its inactive state, acts as a cofactor in the intrinsic pathway.
- When activated to VIIIa, it enhances the activity of factor IXa in the tenase complex.
VIIIa
- Factor VIIIa is the active cofactor that amplifies the action of factor IXa in the intrinsic pathway.
- It is a critical component of the tenase complex, facilitating factor X activation.
X
- Factor X, in its inactive form, is a convergence point for both intrinsic and extrinsic pathways.
- Activation to Xa is a pivotal step leading to the common pathway.
Xa
- Factor Xa, the active form of factor X, is a serine protease that converts prothrombin to thrombin.
- It plays a central role in the final common pathway of coagulation.
VII
- Factor VII, in its inactive state, is activated by tissue factor in the extrinsic pathway.
- The activation to VIIa initiates the extrinsic coagulation cascade.
VIIa
- Factor VIIa is the active form of factor VII, forming a complex with tissue factor.
- This complex activates factor X, linking the extrinsic pathway to the common pathway.
III
- Factor III, also known as tissue factor, is released upon trauma to extravascular cells.
- It binds with factor VIIa to activate factor X in the extrinsic pathway.
Va
- Factor Va is the active cofactor that enhances the activity of factor Xa in the prothrombinase complex.
- It accelerates the conversion of prothrombin to thrombin.
Prothrombin (II)
- Prothrombin, or factor II, is an inactive precursor protein in the plasma.
- It is converted to thrombin (IIa) by factor Xa in the presence of factor Va.
Thrombin (IIa)
- Thrombin, the active form of prothrombin, is a key enzyme that cleaves fibrinogen into fibrin.
- It also activates factors V, VIII, and XIII, amplifying the coagulation process.
Fibrinogen (I)
- Fibrinogen is a soluble plasma protein that serves as the precursor to fibrin.
- It is converted to fibrin by thrombin, forming the structural basis of a blood clot.
Fibrin (Ia)
- Fibrin is the insoluble protein formed from fibrinogen, creating a mesh that stabilizes the clot.
- It is cross-linked by factor XIIIa to form a strong, stable clot.
XIIIa
- Factor XIIIa is the active form of factor XIII, responsible for cross-linking fibrin strands.
- This cross-linking enhances the clot’s durability and resistance to breakdown.
Cross-linked fibrin clot
- The cross-linked fibrin clot is the final product of the coagulation cascade, sealing the damaged vessel.
- It provides a stable structure that supports tissue repair and prevents further bleeding.
Factor: inactive state
- The inactive state represents clotting factors before activation, requiring specific triggers to initiate the cascade.
- These factors remain dormant until activated by injury or other stimuli.
Factor: active state
- The active state indicates clotting factors that have been enzymatically activated to perform their roles.
- Activation transforms these factors into catalysts that drive the coagulation process.
The fibrin synthesis cascade is a vital component of hemostasis, orchestrating the transformation of blood into a clot to repair vascular injuries. The diagram illustrates two primary initiation pathways—the intrinsic pathway, triggered by damage to the vessel wall, and the extrinsic pathway, activated by trauma to extravascular cells—both converging into a final common pathway. This process involves a series of enzymatic reactions that ultimately produce a stable fibrin clot, essential for stopping bleeding and supporting tissue healing.
Intrinsic Pathway: Internal Activation of Coagulation
The intrinsic pathway begins with internal damage, setting off a chain of reactions within the blood. This pathway relies on factors already present in the circulation to initiate clotting.
- Damage to the vessel wall activates factor XII, starting the cascade with the conversion to XIIa.
- Subsequent activations of factors XI, IX, and VIII lead to the formation of the tenase complex, which activates factor X.
Extrinsic Pathway: External Trigger for Clotting
The extrinsic pathway is rapidly initiated by external trauma, providing an immediate response to injury. This pathway involves tissue factor to accelerate the coagulation process.
- Trauma to extravascular cells releases factor III, which complexes with factor VIIa to activate factor X.
- This quick activation ensures a prompt clotting response to external damage.
Final Common Pathway: Convergence and Clot Formation
The final common pathway integrates the intrinsic and extrinsic routes, leading to the formation of a stable clot. This stage is where the cascade culminates in fibrin production.
- Factor Xa, generated from both pathways, converts prothrombin to thrombin in the prothrombinase complex with factor Va.
- Thrombin then cleaves fibrinogen into fibrin, which is cross-linked by factor XIIIa to form a cross-linked fibrin clot.
The fibrin synthesis cascade is a meticulously regulated process that ensures effective hemostasis following vascular injury. The intrinsic pathway is activated when the vessel wall is damaged, exposing collagen that triggers factor XII to become XIIa. This activation initiates a series of conversions—XI to XIa, IX to IXa, and VIII to VIIIa—culminating in the tenase complex, which includes IXa and VIIIa. This complex efficiently activates factor X, marking the transition to the common pathway. The process involves a delicate balance, as excessive or insufficient clotting can lead to thrombosis or hemorrhage, respectively.
The extrinsic pathway responds to trauma outside the blood vessels, where extravascular cells release factor III (tissue factor). This factor binds with factor VII, activating it to VIIa, which then activates factor X. The rapid nature of this pathway makes it crucial for immediate clot formation in acute injuries. Both pathways converge at factor X, which, when activated to Xa, forms the prothrombinase complex with factor Va. This complex converts prothrombin (factor II) into thrombin (IIa), a pivotal enzyme in the cascade.
Thrombin plays a multifaceted role, cleaving fibrinogen (factor I) into fibrin (Ia), the building block of the clot. It also activates factor V to Va and factor XIII to XIIIa, enhancing the coagulation process. Factor XIIIa cross-links fibrin strands, creating a cross-linked fibrin clot that is resistant to premature breakdown. The diagram’s depiction of inactive and active states of factors underscores the enzymatic activation required at each step, ensuring a controlled response. This cascade is tightly regulated by natural anticoagulants like protein C and S to prevent unwanted clot formation.
The clinical relevance of the fibrin synthesis cascade extends to conditions involving abnormal clotting. Deficiencies in factors, such as hemophilia A (factor VIII deficiency) or hemophilia B (factor IX deficiency), impair the intrinsic pathway, leading to excessive bleeding. Conversely, excessive activity, as seen in disseminated intravascular coagulation (DIC), can cause widespread clotting and organ damage. Understanding these pathways aids in diagnosing and managing such disorders, often through factor replacement therapy or anticoagulants like heparin.
The interplay of the intrinsic and extrinsic pathways highlights the body’s adaptability to different types of injury. The final common pathway’s reliance on thrombin and fibrin ensures a universal mechanism for clot stabilization, regardless of the initial trigger. This knowledge is foundational for interpreting coagulation tests, such as the activated partial thromboplastin time (aPTT) for the intrinsic pathway and prothrombin time (PT) for the extrinsic pathway. Mastery of this cascade enhances the ability to address both routine and critical care scenarios effectively.
The fibrin synthesis cascade exemplifies the body’s remarkable capacity to protect itself from hemorrhage. By integrating the intrinsic and extrinsic pathways into a cohesive final common pathway, the process ensures rapid and stable clot formation. This understanding not only deepens appreciation for hemostatic mechanisms but also supports informed clinical decision-making.

