The kidneys possess an intricate self-regulatory mechanism to maintain stable blood filtration, primarily orchestrated by the Juxtaglomerular Apparatus (JGA). This article explores the detailed anatomical structure of the JGA and the glomerulus, highlighting how this specialized cellular complex monitors filtrate composition and precisely adjusts the glomerular filtration rate. Understanding the JGA’s role is fundamental to comprehending blood pressure regulation, electrolyte balance, and the pathophysiology of many renal conditions.
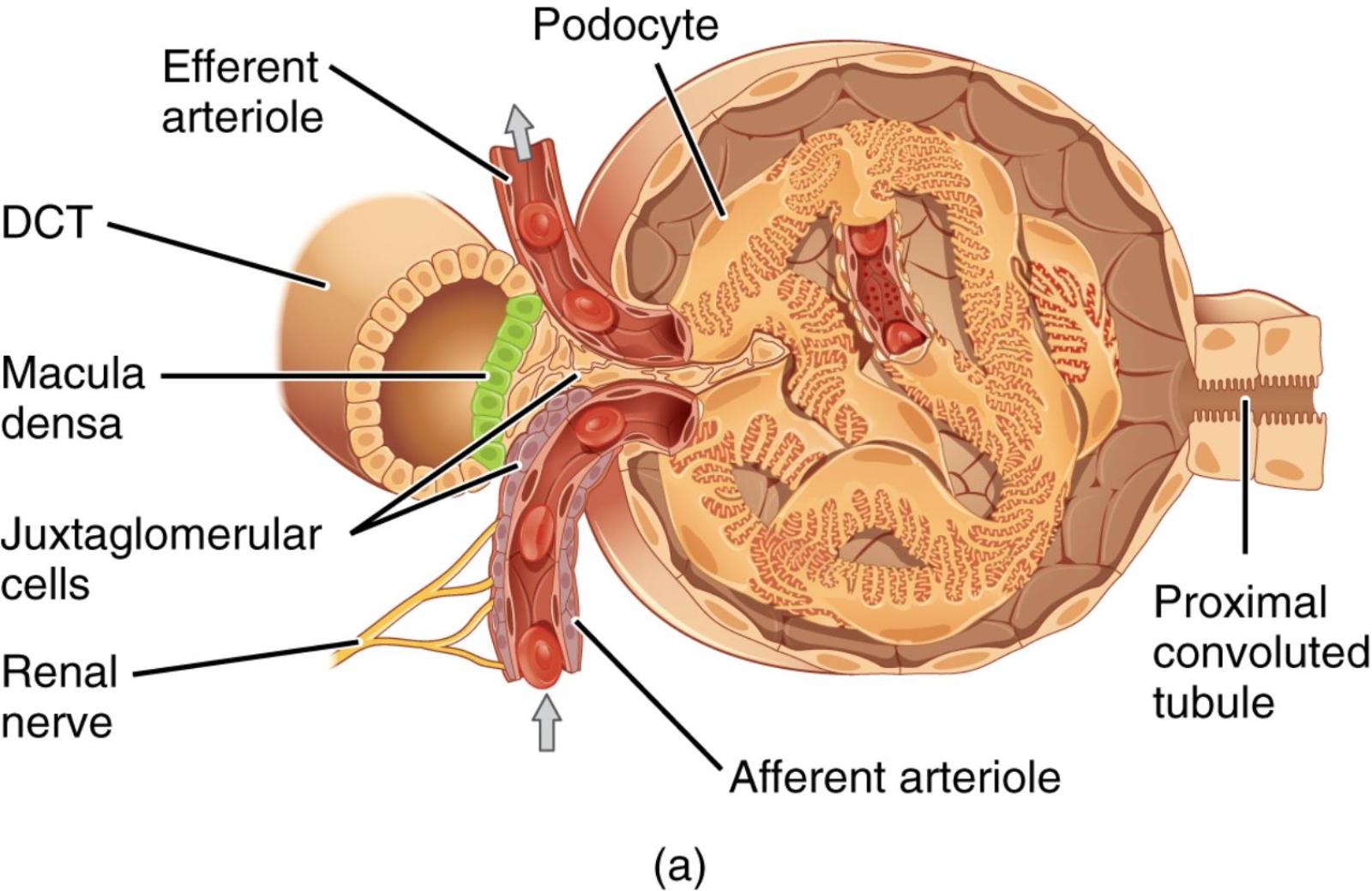
Efferent arteriole: This small artery carries blood away from the glomerulus after initial filtration has occurred. Its diameter can be regulated to influence pressure within the glomerulus and peritubular capillaries.
DCT (Distal Convoluted Tubule): This segment of the renal tubule follows the loop of Henle and plays a crucial role in fine-tuning ion and water reabsorption. It is here that the macula densa cells of the JGA monitor filtrate composition.
Macula densa: These are specialized chemoreceptor cells located in the wall of the distal convoluted tubule where it contacts the afferent and efferent arterioles. They sense changes in sodium chloride concentration in the filtrate and signal the juxtaglomerular cells.
Juxtaglomerular cells (also known as granular cells): These are specialized smooth muscle cells in the wall of the afferent arteriole, adjacent to the macula densa. They synthesize, store, and secrete renin, an enzyme crucial for blood pressure regulation.
Renal nerve: These nerves, primarily sympathetic, innervate the afferent and efferent arterioles and the juxtaglomerular cells. They modulate renal blood flow and renin release in response to systemic physiological demands.
Afferent arteriole: This small artery carries unfiltered blood into the glomerulus. Its diameter is regulated by various mechanisms, including signals from the JGA, to control glomerular hydrostatic pressure.
Podocyte: These are specialized epithelial cells that line the outer surface of the glomerular capillaries, forming part of the filtration barrier. Their interdigitating foot processes create filtration slits, allowing for selective passage of substances.
Proximal convoluted tubule: This is the initial segment of the renal tubule, where the majority of filtered substances like glucose, amino acids, and water are reabsorbed from the filtrate. It receives the filtrate directly from the glomerular capsule.
The glomerulus, the initial filtering unit of the kidney, works in concert with a remarkable feedback mechanism known as the Juxtaglomerular Apparatus. This specialized cellular complex, strategically positioned where the distal convoluted tubule loops back to touch the renal corpuscle, acts as the kidney’s intrinsic regulator of blood flow and filtration rate. It ensures that the glomerular filtration rate (GFR) remains stable despite fluctuations in systemic blood pressure, and it plays a critical role in the body’s long-term control of blood pressure and fluid balance. The intricate anatomical arrangement shown in the image highlights the close communication between the vascular and tubular components of the nephron, which is essential for this precise regulatory function.
The Juxtaglomerular Apparatus (JGA) consists of three main cell types: the macula densa cells, the juxtaglomerular (granular) cells, and extraglomerular mesangial cells (not explicitly labeled but present in the region). The macula densa cells, located in the wall of the distal convoluted tubule, are chemoreceptors that sense the sodium chloride concentration in the tubular fluid. A decrease in NaCl delivery or concentration at the macula densa typically indicates a lower GFR. In response to these changes, the macula densa cells release paracrine signals, primarily prostaglandins, that affect the adjacent afferent arteriole and juxtaglomerular cells. This communication is vital for the tubuloglomerular feedback mechanism.
The juxtaglomerular cells, specialized smooth muscle cells in the wall of the afferent arteriole, are mechano-receptors that detect changes in blood pressure within the arteriole. They are also the primary site for the synthesis and secretion of renin, a crucial enzyme in the renin-angiotensin-aldosterone system (RAAS). When blood pressure in the afferent arteriole drops, or when the macula densa signals low NaCl, the juxtaglomerular cells release renin. Renin initiates a cascade that ultimately leads to increased blood pressure and sodium reabsorption, thereby helping to restore GFR and systemic blood pressure. Furthermore, the renal nerves, part of the sympathetic nervous system, also directly innervate the afferent arteriole and juxtaglomerular cells, providing an additional layer of extrinsic control over renal blood flow and renin release, especially in response to stress or significant changes in systemic blood volume.
Dysfunction of the Juxtaglomerular Apparatus can have profound effects on renal function and systemic blood pressure regulation. For example, conditions like renal artery stenosis, which reduces blood flow to the kidney, will activate the JGA, leading to excessive renin release and secondary hypertension. Conversely, damage to the macula densa or juxtaglomerular cells can impair the kidney’s ability to autoregulate GFR and maintain electrolyte balance. Understanding the detailed anatomy and physiological roles of each component of the JGA is therefore critical for diagnosing and managing various hypertensive disorders, electrolyte imbalances, and progressive kidney diseases. This complex feedback system underscores the kidney’s remarkable capacity for self-regulation, ensuring optimal filtration and maintaining overall body homeostasis.

