Explore the crucial role of temperature in modulating oxygen delivery within the body, as illustrated by its effect on the oxygen-hemoglobin dissociation curve. This article details how variations in body temperature influence hemoglobin’s affinity for oxygen, ensuring efficient oxygen release to metabolically active, warmer tissues.
Temperature’s Dynamic Role in Oxygen Transport
The precise delivery of oxygen to the body’s tissues is a finely tuned physiological process, influenced by a myriad of factors beyond just the partial pressure of oxygen (PO2). Among these, body temperature plays a significant and dynamic role in regulating how tightly hemoglobin binds to oxygen. This intricate relationship is graphically represented by the oxygen-hemoglobin dissociation curve, which shifts in response to changes in temperature, a crucial adaptation for meeting the metabolic demands of various tissues.
Understanding how temperature affects hemoglobin’s affinity for oxygen is fundamental in medical science. It helps explain physiological responses in conditions ranging from strenuous exercise to hypothermia and hyperthermia. This temperature-dependent modulation ensures that oxygen is efficiently unloaded where it is most needed, particularly in metabolically active areas that generate more heat.
Key insights into temperature’s effect on oxygen transport include:
- Increased temperature promotes oxygen release to tissues.
- Decreased temperature enhances oxygen binding in cooler environments.
- A physiological mechanism for targeted oxygen delivery.
These adaptations are vital for maintaining cellular function and overall bodily homeostasis.
The Effect of Temperature on Oxygen-Hemoglobin Affinity
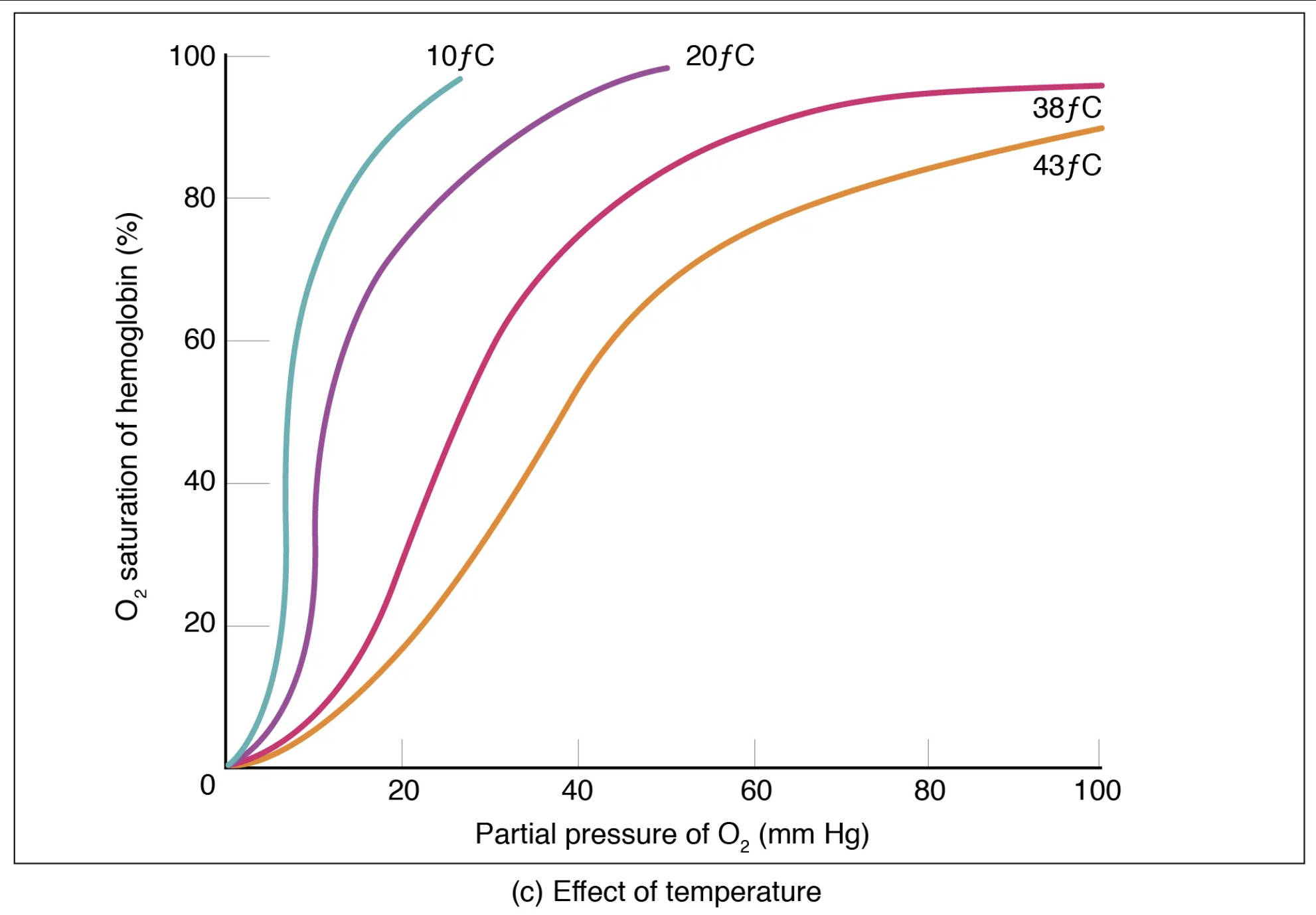
The oxygen-hemoglobin dissociation curve plots the percentage of hemoglobin saturation with oxygen against the partial pressure of oxygen (PO2). This curve demonstrates how hemoglobin’s affinity for oxygen changes with varying PO2. However, temperature significantly influences this affinity, causing the curve to shift.
O2 saturation of hemoglobin (%): This vertical axis indicates the percentage of hemoglobin molecules that are currently bound to oxygen. A higher percentage signifies that more oxygen is being carried by the blood.
Partial pressure of O2 (mm Hg): This horizontal axis represents the partial pressure of oxygen, which is the driving force for oxygen to bind to or dissociate from hemoglobin. Higher partial pressures, as in the lungs, encourage oxygen loading.
10°C: This curve, shifted significantly to the left, represents a very low temperature. At this temperature, hemoglobin has a very high affinity for oxygen, binding it tightly and releasing very little to the tissues, even at lower PO2 levels. This would be observed in severe hypothermia.
20°C: This curve, also shifted to the left compared to normal body temperature, indicates a higher affinity for oxygen than at warmer temperatures. Hemoglobin binds oxygen more readily at 20°C than at 38°C or 43°C.
38°C: This curve represents a temperature slightly above normal body temperature, indicating a moderate affinity for oxygen. This curve is positioned slightly to the right of the 37°C curve (normal body temp), showing a tendency to release oxygen more readily.
43°C: This curve, shifted furthest to the right, represents a significantly elevated temperature. At this temperature, hemoglobin’s affinity for oxygen is considerably reduced, causing it to release oxygen much more readily to the tissues, even at higher PO2 levels. This is typically seen in fever or intensely active tissues.
Temperature and Oxygen Unloading: A Physiological Advantage
The effect of temperature on the oxygen-hemoglobin dissociation curve is a crucial physiological adaptation. As body temperature increases, the oxygen-hemoglobin dissociation curve shifts to the right. This rightward shift indicates that hemoglobin’s affinity for oxygen decreases, meaning it releases oxygen more readily to the surrounding tissues for any given partial pressure of oxygen. This mechanism is incredibly advantageous in situations where tissues have increased metabolic activity, such as during strenuous exercise. Actively working muscles generate more heat, leading to a localized increase in temperature. This localized warming acts as a signal, prompting hemoglobin to unload more oxygen precisely where it is most needed to support ongoing cellular respiration and energy production.
Conversely, when body temperature decreases (e.g., in cooler peripheral tissues or during hypothermia), the oxygen-hemoglobin dissociation curve shifts to the left. This leftward shift signifies that hemoglobin’s affinity for oxygen increases, causing it to bind oxygen more tightly and release less of it to the tissues. While this might seem counterproductive, it helps to preserve oxygen in the blood when metabolic demands are lower. In conditions of systemic hypothermia, this increased oxygen affinity can become problematic, as it hinders oxygen release to vital organs, potentially leading to tissue hypoxia despite adequate arterial oxygenation.
Clinical Implications of Temperature Shifts
The temperature-dependent shifts in the oxygen-hemoglobin dissociation curve have significant clinical implications. In patients with fever, the increased body temperature (e.g., 38°C to 43°C) leads to a rightward shift, enhancing oxygen unloading to fight infection and support increased metabolic rates. However, in severe hyperthermia, this can be extreme, potentially compromising oxygen loading in the lungs if temperatures are very high.
In hypothermia, as seen in accidental cold exposure or medically induced cooling, the leftward shift of the curve can reduce oxygen availability to tissues, even if the blood is saturated with oxygen. This is a critical consideration in managing patients undergoing therapeutic hypothermia after cardiac arrest or brain injury, where careful monitoring of tissue oxygenation is essential. Understanding the effect of temperature on hemoglobin’s oxygen affinity allows clinicians to better interpret blood gas results and implement appropriate interventions to optimize oxygen delivery in various clinical scenarios.
Conclusion
The influence of temperature on the oxygen-hemoglobin dissociation curve highlights another elegant regulatory mechanism within the human body. This dynamic relationship ensures that oxygen is efficiently released to metabolically active, warmer tissues and effectively loaded in cooler environments, adapting to varying physiological demands. A comprehensive understanding of how temperature modulates hemoglobin’s affinity for oxygen is indispensable for medical professionals in interpreting physiological responses and managing a wide array of conditions that impact oxygen transport, ultimately playing a critical role in maintaining cellular viability and patient well-being.

