The kidney’s ability to precisely filter blood relies on highly specialized cells known as podocytes. This article delves into the intricate anatomical structure of podocytes, explaining how their unique morphology forms a crucial part of the glomerular filtration barrier. Understanding the function of podocytes and the delicate filtration slits they create is essential for comprehending renal physiology and the pathology of various kidney
diseases.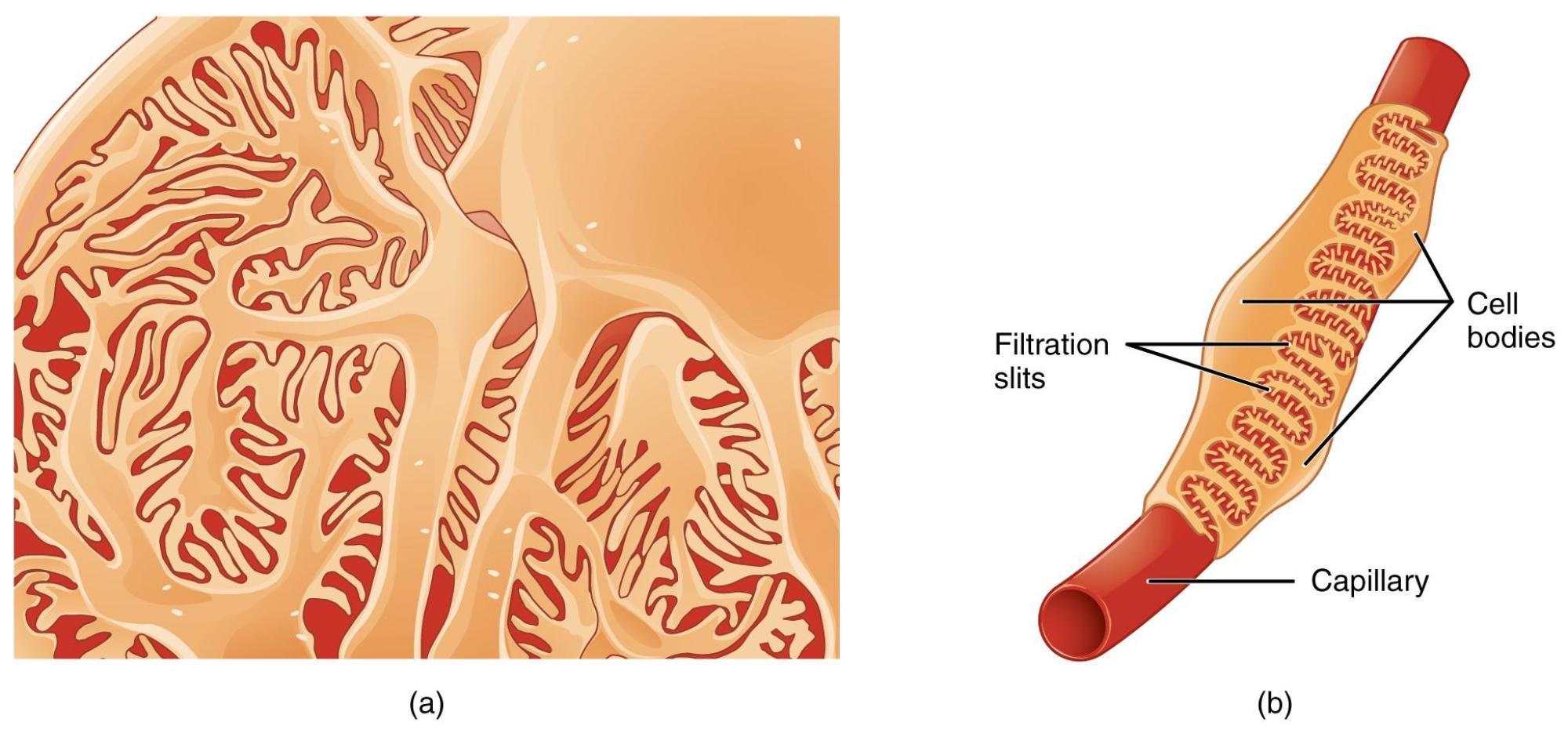
Filtration slits: These are narrow gaps or pores formed between the interdigitating pedicels of adjacent podocytes. They represent the final barrier that solutes must cross to become part of the glomerular filtrate.
Cell bodies: These are the main parts of the podocyte cells, containing the nucleus and other organelles. From the cell body, primary processes extend, which then branch into secondary processes and ultimately the pedicels.
Capillary: This refers to the glomerular capillary, a specialized blood vessel around which the podocytes are wrapped. It is within these capillaries that blood plasma is initially filtered to form urine.
The glomerulus, the initial filtering unit of the kidney, is a marvel of biological engineering, meticulously designed to separate waste products from the blood while retaining essential proteins and cells. At the heart of this filtration process are podocytes, uniquely shaped cells that line the outer surface of the glomerular capillaries. Their intricate structure, characterized by numerous finger-like projections, creates a highly selective barrier that allows for efficient filtration. This image provides a detailed view of these extraordinary cells, revealing how their interdigitating foot processes form the crucial filtration slits.
Podocytes are epithelial cells that form the visceral layer of Bowman’s capsule, directly wrapping around the glomerular capillaries. Each podocyte possesses a large cell body from which several primary processes extend. These primary processes then branch into numerous secondary processes, which further subdivide into thousands of tiny, foot-like projections called pedicels. These pedicels interdigitate with those from adjacent podocytes, forming a sieve-like structure with narrow gaps known as filtration slits. These slits are not merely passive openings; they are spanned by a specialized protein complex called the slit diaphragm, which acts as the ultimate size-selective barrier, preventing the passage of large molecules like proteins into the urinary space.
The primary function of podocytes and their filtration slits is to regulate the passage of molecules from the blood into the glomerular filtrate. Along with the fenestrated endothelium of the glomerular capillaries and the glomerular basement membrane, podocytes form the three layers of the glomerular filtration barrier. This barrier is highly permeable to water and small solutes but largely impermeable to larger molecules, such as plasma proteins and blood cells. The precise spacing and molecular composition of the slit diaphragm are crucial for preventing proteinuria, a condition where excess protein is found in the urine, indicating damage to this delicate filtration system. The negative charges on the surface of the podocytes and the slit diaphragm also repel negatively charged proteins, further enhancing the selectivity of the filter.
Dysfunction or damage to podocytes is a hallmark of many debilitating kidney diseases, collectively known as podocytopathies. Conditions like focal segmental glomerulosclerosis (FSGS), minimal change disease, and diabetic nephropathy often involve structural or functional alterations in podocytes. When podocytes are damaged, their intricate foot processes can efface (flatten and merge), leading to the widening of filtration slits and a breakdown of the slit diaphragm. This compromises the integrity of the filtration barrier, resulting in increased permeability to proteins and leading to significant proteinuria, a key indicator of renal pathology. Understanding the morphology and molecular biology of podocytes is therefore critical for developing targeted therapies for these progressive kidney diseases, ultimately aiming to preserve glomerular function and prevent end-stage renal failure.

