Explore the intricate world of lipid metabolism, a complex network of biochemical pathways governing the synthesis and breakdown of fats in the body. This vital process ensures efficient energy storage, provides structural components for cell membranes, and generates signaling molecules essential for overall health.
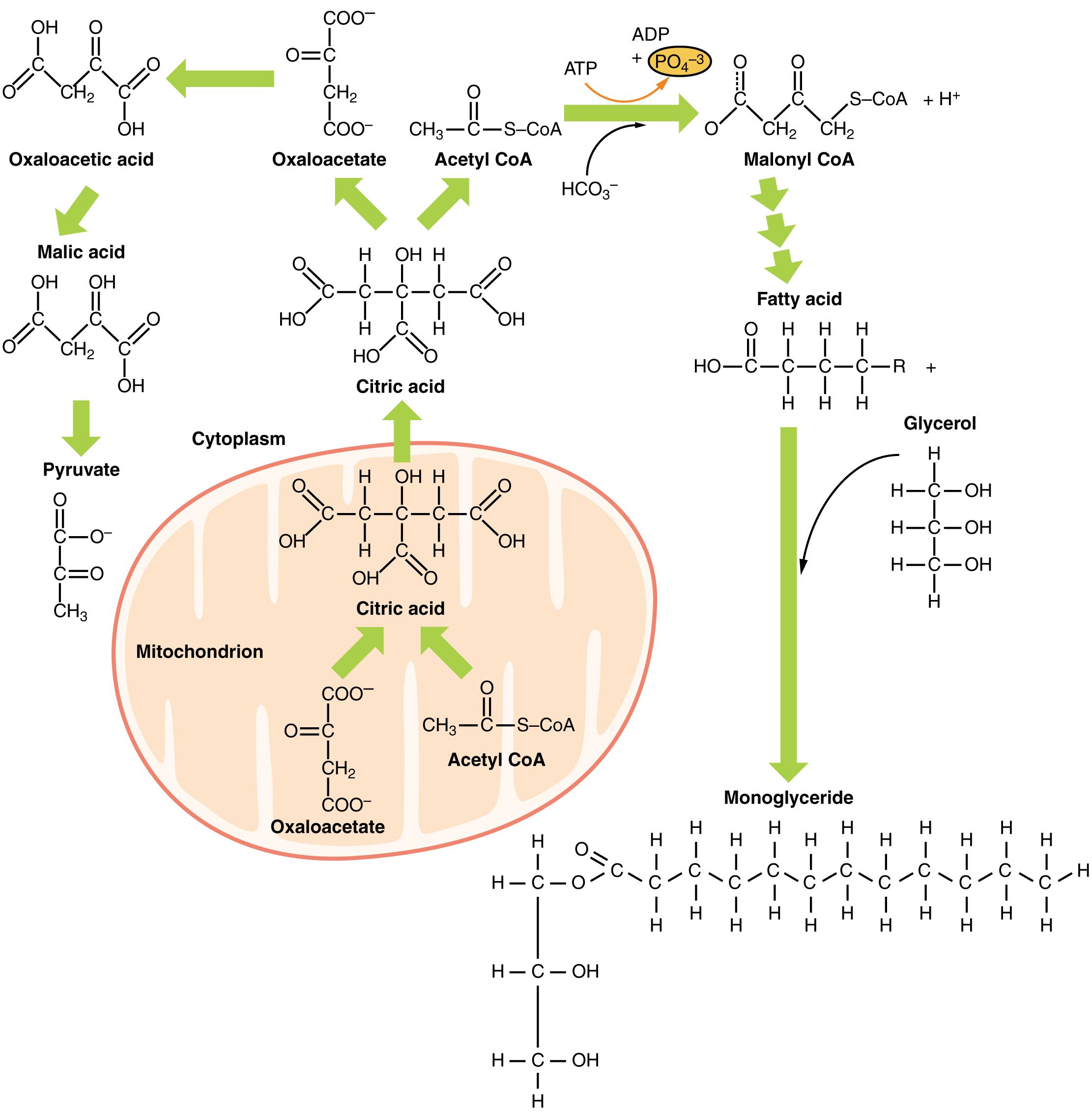
Understanding the Lipid Metabolism Diagram
Oxaloacetic acid: This is an α-keto dicarboxylic acid, a key intermediate in the Krebs cycle and gluconeogenesis. In the context of lipid synthesis, oxaloacetate is converted to malate, which can then generate NADPH for fatty acid synthesis in the cytoplasm.
Malic acid: This dicarboxylic acid is an intermediate in the Krebs cycle and also plays a role in shuttling carbon atoms from the mitochondrion to the cytoplasm for fatty acid synthesis. In the cytoplasm, malate can be decarboxylated by malic enzyme to produce pyruvate and NADPH, a crucial reducing equivalent for anabolic reactions.
Pyruvate: A three-carbon alpha-keto acid that is the end product of glycolysis. Pyruvate can be converted to acetyl CoA, which is a central precursor for fatty acid synthesis when glucose levels are high.
Cytoplasm: The jelly-like substance that fills the cell and surrounds the organelles. Many of the initial steps of lipid synthesis, particularly fatty acid synthesis from acetyl CoA, occur in the cytoplasm.
Mitochondrion: An organelle responsible for cellular respiration and energy production. In lipid metabolism, the mitochondrion is the primary site for the oxidation (breakdown) of fatty acids, but it also plays a role in transporting intermediates for synthesis.
Citric acid: This six-carbon tricarboxylic acid is the first intermediate in the Krebs cycle. In lipid synthesis, citrate is transported from the mitochondrion to the cytoplasm, where it can be cleaved to provide acetyl CoA for fatty acid synthesis.
Oxaloacetate: A four-carbon dicarboxylic acid that condenses with acetyl CoA to form citrate in the Krebs cycle. It is also regenerated at the end of the Krebs cycle, allowing the cycle to continue.
Acetyl CoA: Acetyl coenzyme A is a central molecule in metabolism, connecting carbohydrate, fat, and protein metabolism. It is the primary building block for fatty acid and cholesterol synthesis.
ATP: Adenosine triphosphate, the primary energy currency of the cell. ATP is consumed in various steps of lipid synthesis, indicating that these are energy-requiring anabolic processes.
PO4 3- (Inorganic Phosphate): This is a free phosphate group, often released during energy-consuming reactions where ATP is hydrolyzed. Its presence indicates an energy input for a specific metabolic step.
HCO3-: Bicarbonate ion, which serves as the source of CO2 for the carboxylation of acetyl CoA to malonyl CoA. This carboxylation step is a crucial and rate-limiting step in fatty acid synthesis.
Malonyl CoA: A three-carbon molecule formed by the carboxylation of acetyl CoA. Malonyl CoA is a key intermediate in fatty acid synthesis and also acts as a regulatory molecule, inhibiting the carnitine shuttle to prevent simultaneous fatty acid synthesis and breakdown.
Fatty acid: A carboxylic acid with a long aliphatic chain, which can be saturated or unsaturated. Fatty acids are synthesized from malonyl CoA units and are stored as triglycerides or incorporated into phospholipids.
Glycerol: A three-carbon alcohol that forms the backbone of triglycerides and phospholipids. In lipid metabolism, glycerol can be derived from the breakdown of triglycerides or synthesized from glycolytic intermediates.
Monoglyceride: A molecule composed of a glycerol backbone with only one fatty acid attached. Monoglycerides are formed during the digestion of triglycerides or can be an intermediate in their synthesis.
The Dynamic Interplay of Lipid Pathways
Lipid metabolism encompasses a broad spectrum of biochemical processes that govern the synthesis, breakdown, and transport of lipids within the body. These pathways are crucial for maintaining cellular integrity, providing concentrated energy reserves, and generating essential signaling molecules. Unlike carbohydrates and proteins, lipids are structurally diverse, leading to a variety of metabolic fates for their different components, such as glycerol and fatty acids. This intricate network ensures that the body can adapt to varying nutritional states, efficiently storing excess energy or mobilizing reserves when fuel is scarce.
At the heart of lipid metabolism lies the balance between anabolism (synthesis) and catabolism (breakdown). When energy intake exceeds expenditure, excess carbohydrates and proteins can be converted into fats and stored, primarily as triglycerides in adipose tissue. Conversely, during periods of fasting or increased energy demand, these stored triglycerides are hydrolyzed, releasing fatty acids and glycerol to be oxidized for ATP production. This dynamic interplay is tightly regulated by hormones such as insulin, glucagon, and adrenaline, which signal the body’s energy status and direct metabolic flow.
The liver plays a central role in lipid metabolism, serving as the primary site for fatty acid synthesis, cholesterol synthesis, and the packaging of lipids into lipoproteins for transport to other tissues. Adipose tissue, on the other hand, is specialized for efficient triglyceride storage and release. These tissues work in concert, alongside the small intestine (for dietary fat absorption) and peripheral tissues (for fat utilization), to ensure that the body’s lipid requirements are met while maintaining metabolic homeostasis. Understanding these pathways is fundamental to comprehending metabolic diseases such as obesity, diabetes, and cardiovascular disorders.
- Lipid metabolism involves synthesis, breakdown, and transport of fats.
- It ensures energy storage and provides structural components.
- Glycerol and fatty acids follow distinct metabolic routes.
- The liver and adipose tissue are key players.
Divergent Paths: Glycerol and Fatty Acid Metabolism
The breakdown of triglycerides initiates the divergence of its two main components: glycerol and fatty acids. Glycerol, being a three-carbon alcohol, is water-soluble and can be readily transported to the liver. Once in the liver, glycerol is phosphorylated to glycerol-3-phosphate, an ATP-dependent reaction. This glycerol-3-phosphate can then be isomerized to dihydroxyacetone phosphate (DHAP), a glycolytic intermediate. This allows glycerol to enter the glycolysis pathway and be converted into pyruvate, or it can be used for gluconeogenesis to synthesize glucose, or even re-esterified to form new triglycerides. This metabolic flexibility highlights glycerol’s crucial role in bridging fat and carbohydrate metabolism.
Fatty acids, in contrast to glycerol, follow a distinct and more complex catabolic pathway known as beta-oxidation. Long-chain fatty acids are first activated to fatty acyl CoA in the cytoplasm and then transported into the mitochondrial matrix via the carnitine shuttle system. Inside the mitochondria, beta-oxidation systematically breaks down the fatty acyl CoA into two-carbon units of acetyl CoA. Each cycle of beta-oxidation also produces NADH and FADH2, high-energy electron carriers that feed into the electron transport chain to generate substantial amounts of ATP. The resulting acetyl CoA then enters the Krebs cycle for further oxidation, underscoring the high energy yield from fatty acid breakdown.
The Anabolic Pathways: Lipid Synthesis
When energy is abundant, the body shifts towards lipid synthesis, converting excess calories into fat for storage. The synthesis of fatty acids occurs primarily in the cytoplasm of liver cells and adipose tissue. This process starts with acetyl CoA, which is generated from excess glucose (via pyruvate oxidation) or amino acids. However, acetyl CoA produced in the mitochondrion must first be transported to the cytoplasm. This is achieved by condensing acetyl CoA with oxaloacetate to form citrate, which then exits the mitochondrion. In the cytoplasm, citrate is cleaved back into acetyl CoA and oxaloacetate by ATP-citrate lyase.
The cytoplasmic acetyl CoA is then carboxylated to malonyl CoA by acetyl CoA carboxylase, an ATP-dependent and rate-limiting step in fatty acid synthesis. Malonyl CoA, along with subsequent additions of acetyl CoA units, is then used by the fatty acid synthase complex to sequentially build up long-chain fatty acids. NADPH, generated largely by the pentose phosphate pathway and the malic enzyme (converting malate to pyruvate), provides the reducing power for these synthetic reactions. These newly synthesized fatty acids can then combine with glycerol-3-phosphate to form triglycerides, which are then packaged into very low-density lipoproteins (VLDLs) in the liver for transport to adipose tissue for storage.
Clinical Implications of Lipid Dysregulation
Disruptions in lipid metabolism are central to the pathogenesis of many chronic diseases. Dyslipidemia, characterized by abnormal levels of lipids (cholesterol and triglycerides) in the blood, is a major risk factor for atherosclerosis, coronary artery disease, and stroke. Conditions like hypertriglyceridemia, where there is an excessive accumulation of triglycerides, can result from various genetic factors, dietary habits (e.g., high intake of refined carbohydrates), obesity, and metabolic syndrome. Conversely, inherited defects in specific enzymes involved in fatty acid oxidation can lead to severe metabolic disorders, causing issues like hypoglycemia, muscle weakness, and cardiomyopathy, particularly during periods of fasting.
Furthermore, obesity, a global health epidemic, is fundamentally a disorder of lipid metabolism where energy intake consistently exceeds expenditure, leading to excessive triglyceride storage in adipose tissue. This chronic energy surplus can then contribute to insulin resistance, Type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD), illustrating the widespread impact of dysfunctional lipid pathways on overall health. Understanding the complex regulation of lipid synthesis and breakdown is therefore critical for developing effective therapeutic strategies to manage these prevalent and debilitating conditions.
Conclusion
Lipid metabolism represents a fascinating and highly regulated network of biochemical pathways crucial for cellular function and organismal survival. From the divergent catabolism of glycerol and fatty acids to the intricate anabolic processes of lipid synthesis, these pathways ensure energy homeostasis, membrane integrity, and the production of vital signaling molecules. The dynamic interplay between these processes and their hormonal control underpins our understanding of health and disease, offering critical insights into metabolic disorders and guiding the development of therapeutic interventions.

