Ligand-gated channels are essential components of cellular communication, particularly in the nervous system, where they respond to specific molecules like neurotransmitters. This diagram illustrates how acetylcholine, a key neurotransmitter, binds to a channel protein, opening a pore to allow ions such as sodium, calcium, and potassium to pass through, influencing nerve signaling. Delving into this process provides a deeper understanding of how these channels regulate physiological functions and maintain cellular balance.
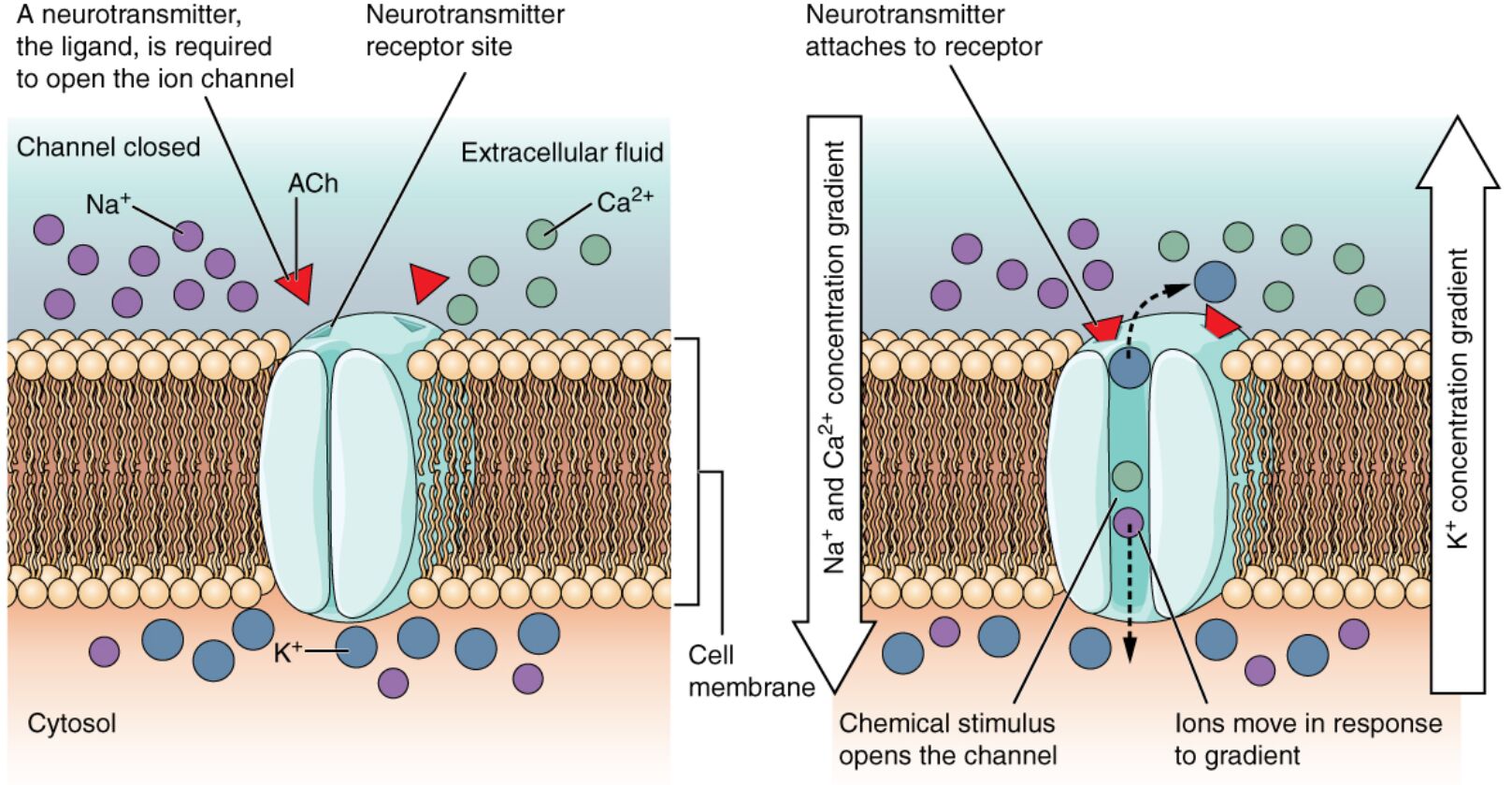
Key Labels in the Ligand-Gated Channels Diagram
This section details each labeled element, shedding light on the mechanism of ion channel activation.
Acetylcholine: This neurotransmitter binds to the receptor site on the ligand-gated channel, triggering a conformational change that opens the pore. It is critical for neuromuscular transmission and autonomic nervous system functions.
Ligand-gated channel: This protein spans the cell membrane, forming a gate that opens upon ligand binding, allowing ion passage. Its activation facilitates rapid signaling between neurons or from neurons to muscles.
Extracellular space: The area outside the cell where acetylcholine is released and binds to the channel, initiating the response. It contains various signaling molecules that interact with the cell surface.
Cytoplasm: The internal environment of the cell where ions enter after passing through the open channel, influencing intracellular processes. It serves as the site where these ions exert their physiological effects.
Channel pore: The selective passageway within the ligand-gated channel that opens to allow specific ions, such as sodium and potassium, to move across the membrane. Its size and structure determine ion specificity and flow rate.
Sodium ion (Na+): This positively charged ion moves into the cell when the channel opens, contributing to depolarization during nerve impulses. Its influx is essential for action potential generation.
Calcium ion (Ca2+): This ion enters the cell upon channel opening, playing a role in neurotransmitter release and muscle contraction. Its presence triggers various intracellular signaling cascades.
Potassium ion (K+): This ion may exit or enter the cell depending on the channel’s properties, helping to repolarize the membrane after an action potential. It maintains the resting membrane potential.
Receptor site: The specific binding location on the ligand-gated channel where acetylcholine attaches, initiating the opening mechanism. Its shape and chemistry ensure selective ligand recognition.
Mechanism of Ligand-Gated Channels
Ligand-gated channels are vital for rapid signal transmission. They respond dynamically to neurotransmitter binding.
- Acetylcholine binds to the receptor site, causing the ligand-gated channel to open.
- The channel pore allows sodium ion (Na+), calcium ion (Ca2+), and potassium ion (K+) to pass.
- This ion movement generates electrical changes across the membrane.
- The process is reversible, closing when acetylcholine dissociates.
- These channels are concentrated at synapses for efficient signaling.
Role of Acetylcholine in Channel Activation
Acetylcholine serves as a key regulator of ligand-gated channels. Its action drives physiological responses.
- Binding of acetylcholine to the receptor site opens the ligand-gated channel.
- This occurs at neuromuscular junctions and parasympathetic nerve endings.
- The neurotransmitter is rapidly broken down by acetylcholinesterase.
- Its action supports muscle contraction and heart rate regulation.
- Dysfunction can lead to conditions like myasthenia gravis.
Ion Movement and Cellular Effects
The flow of ions through the channel pore has significant cellular impacts. It shapes electrical and chemical signaling.
- Sodium ion (Na+) influx depolarizes the membrane, initiating action potentials.
- Calcium ion (Ca2+) entry triggers vesicle release of additional neurotransmitters.
- Potassium ion (K+) efflux helps restore the resting potential.
- Ion gradients are maintained by the sodium-potassium pump.
- Imbalances can affect nerve and muscle function.
Structural and Functional Insights
The ligand-gated channel’s design supports its role in communication. Its location and properties are critical.
- The extracellular space provides the environment for acetylcholine release.
- The cytoplasm receives ions, influencing intracellular calcium levels.
- Channels are pentameric, with multiple subunits forming the channel pore.
- Allosteric modulation can enhance or inhibit channel opening.
- Structural studies aid in designing targeted therapies.
Physiological Significance and Clinical Relevance
Ligand-gated channels influence numerous bodily functions. Their study informs medical practice.
- Acetylcholine-mediated channels are targets for anesthetics and muscle relaxants.
- Sodium ion (Na+) and calcium ion (Ca2+) dysregulation links to epilepsy.
- Potassium ion (K+) imbalances affect cardiac rhythm.
- Nicotinic and muscarinic receptors differ in their ligand-gated channel responses.
- Research into channelopathies guides personalized treatment.
In conclusion, the ligand-gated channels diagram showcases the intricate process where acetylcholine activates the ligand-gated channel, allowing sodium ion (Na+), calcium ion (Ca2+), and potassium ion (K+) to flow through the channel pore. This mechanism underpins rapid communication between cells, from nerve impulses to muscle contraction, highlighting the extracellular space and cytoplasm’s roles. Understanding these dynamics enhances our grasp of cellular physiology and its therapeutic potential.

