Hemostasis is a vital physiological mechanism that prevents excessive blood loss following vascular injury, involving a coordinated series of events to seal damaged vessels. This diagram, credited to Kevin MacKenzie, illustrates the three primary steps of blood clotting—vascular spasm, platelet plug formation, and coagulation—along with the intrinsic and extrinsic pathways leading to fibrin synthesis. Exploring this process provides a deeper appreciation of how the body maintains circulatory integrity and repairs itself after trauma.
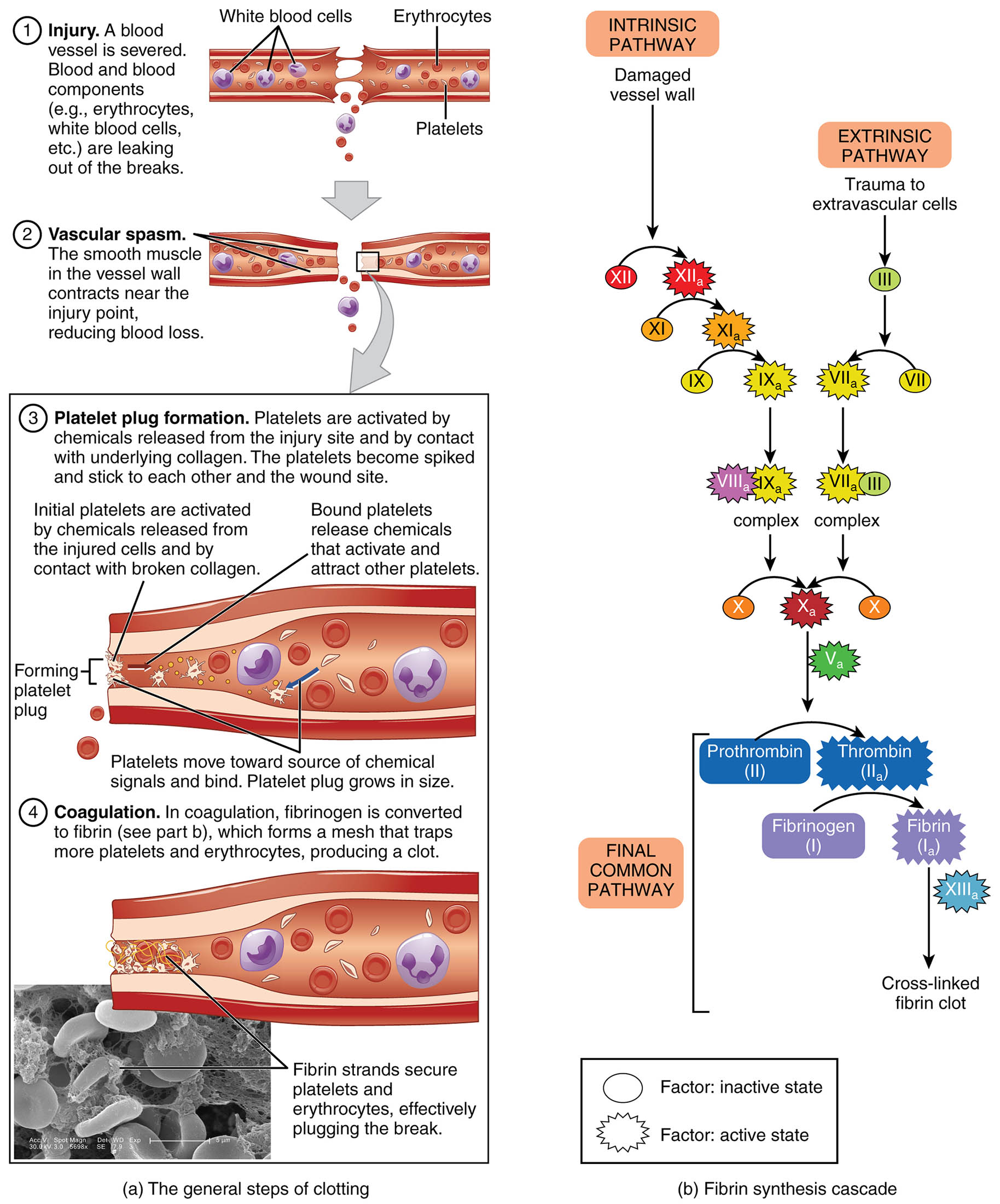
Key Components of the Hemostasis Process
The diagram details the stages and pathways involved in stopping blood loss.
Injured vessel:
The injured vessel is the initial site of damage where a breach in the endothelial lining triggers the hemostasis process. This disruption exposes underlying collagen and tissue factors, initiating a cascade to prevent excessive bleeding.
Vascular spasm:
Vascular spasm involves the immediate constriction of the injured vessel, reducing blood flow to minimize loss and prepare the site for further clotting. This reflex action, mediated by smooth muscle contraction, lasts for several minutes to hours.
Platelet plug:
The platelet plug forms when platelets adhere to the exposed collagen of the injured vessel, aggregating to create a temporary seal for small openings. This initial barrier is stabilized by platelet activation and release of clotting factors.
Coagulation:
Coagulation is the final step where a stable fibrin clot forms to repair the vessel wall after bleeding stops, involving a complex enzymatic cascade. This process ensures long-term sealing and supports tissue healing over time.
Intrinsic pathway:
The intrinsic pathway is activated by contact of blood with negatively charged surfaces, such as damaged endothelium, initiating a series of reactions within the bloodstream. It converges with the extrinsic pathway to amplify the coagulation process.
Extrinsic pathway:
The extrinsic pathway is triggered by the release of tissue factor from injured cells outside the blood vessel, providing a rapid response to external damage. It joins the intrinsic pathway to form the common pathway for fibrin formation.
Common pathway:
The common pathway integrates the intrinsic and extrinsic pathways, leading to the activation of factor X and subsequent thrombin generation. This stage culminates in the conversion of fibrinogen to fibrin, creating a stable clot.
Fibrin:
Fibrin is the insoluble protein mesh that forms the structural basis of the blood clot, produced by the action of thrombin on fibrinogen. It traps red blood cells and platelets, providing strength and durability to the clot during vessel repair.
Thrombin:
Thrombin is a serine protease that converts fibrinogen into fibrin, playing a central role in the coagulation cascade. It also activates other clotting factors and platelets, enhancing the clot’s stability and growth.
The Anatomical and Physiological Role of Hemostasis
Hemostasis is a dynamic process that begins with an injured vessel, where the endothelial lining’s breach exposes subendothelial collagen, triggering vascular spasm to reduce blood flow. This initial response, driven by local neurotransmitters and myogenic reflexes, sets the stage for platelet plug formation, where platelets adhere via glycoprotein Ib and aggregate with the help of von Willebrand factor. Coagulation then ensues, involving the intrinsic pathway (activated by internal blood contact) and extrinsic pathway (triggered by tissue factor), both converging into the common pathway to produce thrombin.
Thrombin catalyzes the conversion of fibrinogen to fibrin, forming a mesh that reinforces the platelet plug and seals the injury. This process is regulated by factors like protein C and antithrombin to prevent excessive clotting, with thyroid hormones (T3 and T4) indirectly influencing metabolic demand that affects clotting efficiency. The resulting fibrin clot supports tissue repair until the vessel heals, showcasing the body’s intricate balance of bleeding control and restoration.
- Spasm Mechanism: Endothelin release constricts vessels; duration depends on injury severity.
- Platelet Activation: ADP and thromboxane A2 enhance aggregation; calcium ions stabilize the plug.
- Coagulation Cascade: Factor VII activates in the extrinsic path; factor XII initiates the intrinsic path.
Physical Characteristics and Clinical Relevance
The physical progression of hemostasis reflects its staged approach, observable through histological analysis. Vascular spasm narrows the vessel lumen, while the platelet plug appears as a dense aggregation under microscopy. The fibrin mesh, stained red with dyes like phosphotungstic acid hematoxylin, forms a robust network, with thrombin activity detectable via clotting assays.
Clinically, this diagram aids in understanding bleeding disorders. Hemophilia, a deficiency in factors of the intrinsic pathway (e.g., factor VIII or IX), leads to prolonged bleeding, treated with factor replacement therapy. Excessive clotting, as in deep vein thrombosis, involves overactive thrombin and fibrin formation, managed with anticoagulants like heparin. Laboratory tests, including prothrombin time (PT) and activated partial thromboplastin time (aPTT), assess pathway function, guiding precise interventions.
- Diagnostic Tools: Thromboelastography measures clot strength; D-dimer tests detect fibrin degradation.
- Therapeutic Options: Vitamin K reverses warfarin effects; tranexamic acid stabilizes clots in trauma.
Conclusion
The hemostasis diagram provides a clear roadmap of how the body swiftly seals injured vessels through vascular spasm, platelet plugs, and fibrin clots, ensuring minimal blood loss and promoting repair. This intricate process, driven by the interplay of intrinsic and extrinsic pathways, highlights the body’s remarkable ability to maintain circulatory stability. By mastering these mechanisms, one can better appreciate the diagnostic and therapeutic strategies that address hemostatic imbalances, underscoring their importance in sustaining health.

