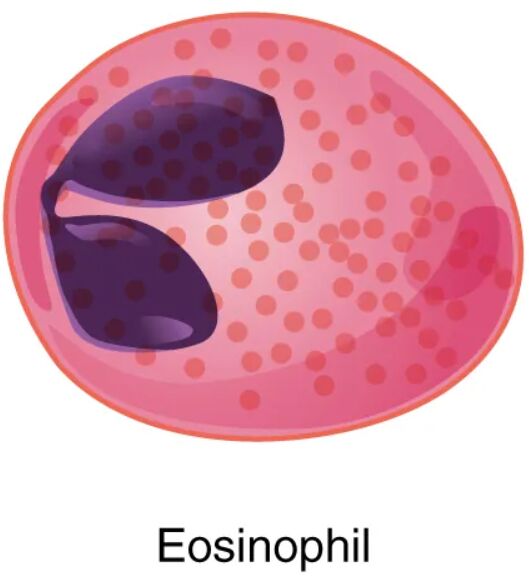
Eosinophils, a type of granular leukocyte, play a vital role in the immune system, particularly in combating parasitic infections and modulating allergic responses. This article examines the structure and function of eosinophils as depicted in the diagram, offering a detailed look at their distinctive features and clinical significance. Understanding these cells enhances insight into their contributions to health and disease.
Cell membrane
- The cell membrane encloses the eosinophil, regulating the entry and exit of substances while maintaining its shape.
- It also contains receptors that detect signals from the immune system, triggering the cell’s activation.
Nucleus
- The nucleus of an eosinophil is typically bi-lobed, containing genetic material that directs the cell’s functions.
- Its segmented structure allows flexibility as the cell moves through tissues to sites of inflammation.
Cytoplasm
- The cytoplasm surrounds the nucleus and houses the cell’s organelles and granules, supporting metabolic activities.
- It provides the medium for intracellular processes, including the storage and release of granular contents.
Granules
- Granules are specialized structures within the cytoplasm, filled with enzymes and proteins like major basic protein and eosinophil peroxidase.
- These granules release their contents to combat parasites or contribute to allergic reactions when the cell is activated.
Major basic protein
- Major basic protein is a key component within eosinophil granules, exhibiting potent antiparasitic properties.
- It is released during immune responses, helping to damage the outer layers of parasites like helminths.
Eosinophil peroxidase
- Eosinophil peroxidase is an enzyme in the granules that generates reactive oxygen species to kill pathogens.
- It works by catalyzing the oxidation of halides, enhancing the cell’s antimicrobial activity.
Eosinophils, as granular leukocytes, are fascinating immune cells with a distinct role in protecting the body from specific threats. The diagram highlights their structure, including the cell membrane, nucleus, cytoplasm, and granules, which contain powerful proteins like major basic protein and eosinophil peroxidase. These components work together to target parasites and mediate allergic and inflammatory responses, making eosinophils essential for maintaining immune balance.
Cellular Structure: Anatomy of the Eosinophil
The eosinophil’s structure is tailored to its immune functions, with each component playing a specific role. This design supports its mobility and effector capabilities.
- The cell membrane protects the cell and facilitates communication with other immune cells.
- The nucleus, with its bi-lobed shape, allows the cell to navigate through tight tissue spaces.
Granular Contents: Functional Proteins
The granules within eosinophils house critical proteins that drive their immune activities. These contents are released strategically during infections or allergic reactions.
- Major basic protein targets and disrupts the integrity of parasitic organisms.
- Eosinophil peroxidase produces toxic compounds that eliminate pathogens effectively.
Eosinophils are a subtype of white blood cells, classified as granular leukocytes due to their prominent cytoplasmic granules, which are stained orange-red with eosin dye—hence their name. The cell membrane encases the cell, featuring receptors for cytokines like interleukin-5 (IL-5), which regulate eosinophil production and activation in the bone marrow. The nucleus, typically divided into two lobes, contains chromatin that directs protein synthesis, adapting the cell for its migratory and combat roles. The cytoplasm serves as a dynamic workspace, housing mitochondria for energy and the characteristic granules that store bioactive molecules.
The granules are the eosinophil’s arsenal, containing major basic protein, which damages the tegument of parasites like Schistosoma or Trichinella, aiding in their destruction. Eosinophil peroxidase, another granule constituent, collaborates with hydrogen peroxide to form hypohalous acids, enhancing the cell’s ability to kill bacteria and fungi. These granules are released via degranulation, a process triggered by immune signals during parasitic infections or allergic conditions like asthma. Eosinophils, produced at a rate of 50-400 million per day under normal conditions, increase significantly during immune challenges, with levels rising from 1-6% of total leukocytes to higher percentages in response to IL-5 stimulation.
Eosinophils are particularly effective against multicellular parasites, releasing major basic protein to disrupt their outer layers, a mechanism less relevant for bacterial infections dominated by neutrophils. The eosinophil peroxidase activity also contributes to oxidative bursts, similar to those in neutrophils, but with a focus on larger pathogens. Their presence in tissues is guided by chemotactic factors, allowing them to accumulate at sites of inflammation, such as the lungs in allergic asthma or the gut in parasitic infections. This targeted migration underscores their role in chronic inflammatory states, where they can both protect and, in excess, contribute to tissue damage.
In allergic conditions, eosinophils are recruited by Th2 lymphocytes, releasing granule contents that promote inflammation, including histamine release from mast cells. This can lead to symptoms like wheezing or eosinophilic esophagitis, where eosinophil infiltration causes tissue remodeling. The cell membrane’s receptors also bind to adhesion molecules on endothelial cells, facilitating extravasation from blood vessels into tissues. The nucleus’s flexibility aids this movement, enabling eosinophils to squeeze through capillary walls, a process enhanced by their amoeboid shape.
Clinically, eosinophil levels are monitored via complete blood counts, with elevations indicating parasitic infections, allergies, or conditions like eosinophilic granulomatosis with polyangiitis. Their role in asthma involves releasing cytokines that sustain airway inflammation, while in hypereosinophilic syndromes, excessive numbers can lead to organ damage. Therapeutic strategies, such as corticosteroids or IL-5 inhibitors (e.g., mepolizumab), target eosinophil activity to manage these conditions. The diagram’s depiction of granule contents highlights their dual nature as both defenders and potential contributors to pathology.
Eosinophils’ interaction with the immune system is a delicate balance, requiring precise regulation. Their ability to release major basic protein and eosinophil peroxidase showcases their specialized defense mechanism, while their involvement in allergies reflects a broader inflammatory role. This understanding not only illuminates their physiological importance but also guides therapeutic interventions for related disorders.

