Bicarbonate Reabsorption in the Proximal Convoluted Tubule: Maintaining Acid-Base Balance
The kidney plays a pivotal role in maintaining the body’s delicate acid-base balance, primarily through its ability to reabsorb bicarbonate (HCO3-) from the filtered fluid. This article details the intricate process of bicarbonate reabsorption from the PCT, illustrating the enzymatic reactions and transport mechanisms involved. Understanding this critical function of the proximal convoluted tubule is fundamental to comprehending systemic pH regulation and the pathophysiology of acid-base disorders.
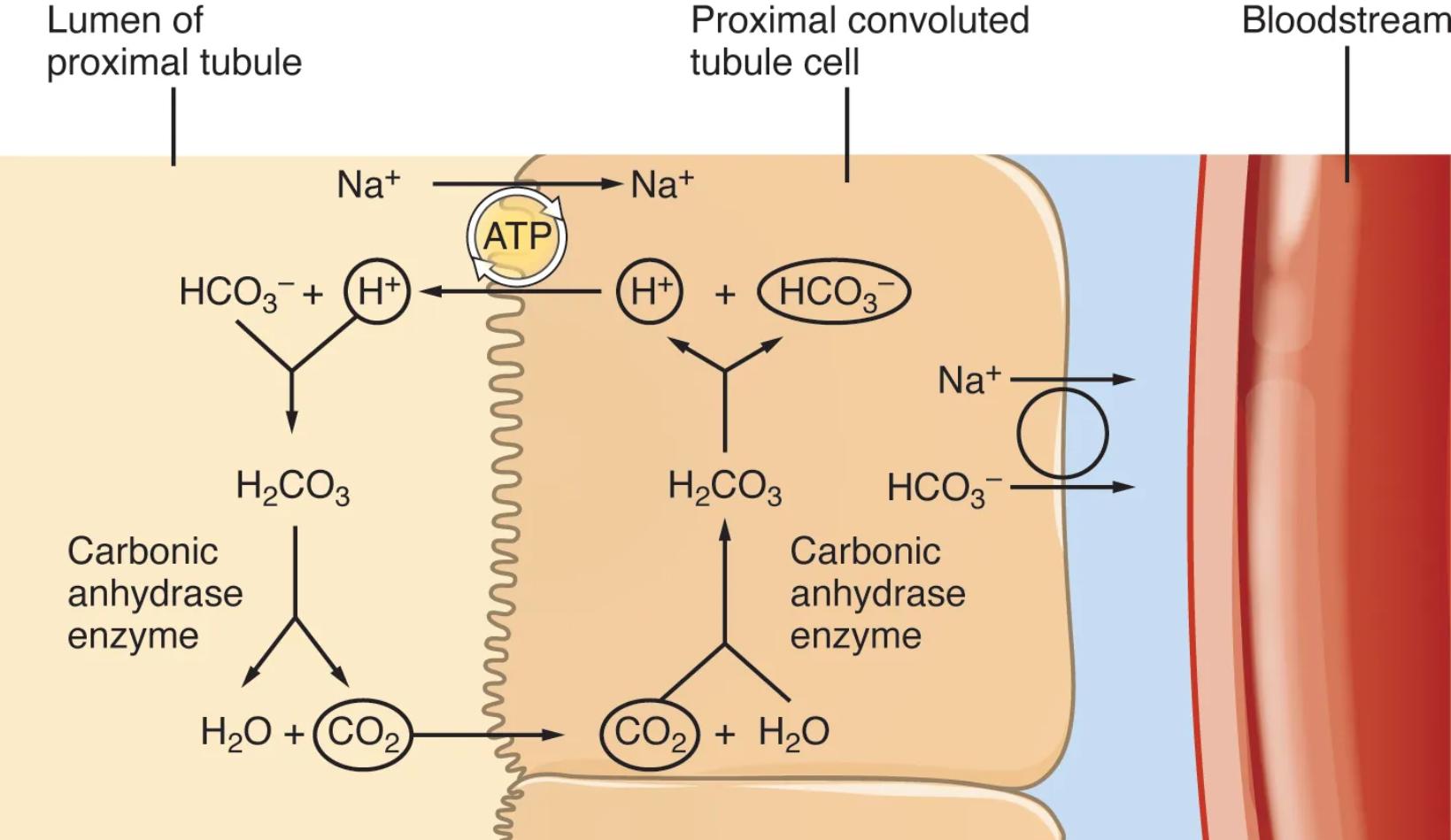
Lumen of proximal tubule: This is the inner space of the proximal convoluted tubule, containing the filtrate that has been formed by the glomerulus. It is from this fluid that bicarbonate is reabsorbed.
Proximal convoluted tubule cell: These are the epithelial cells lining the PCT, specialized for efficient transport processes. They contain the necessary enzymes and transporters to reabsorb bicarbonate.
Bloodstream: This represents the peritubular capillaries, which are blood vessels closely associated with the renal tubules. Reabsorbed bicarbonate ultimately enters the bloodstream to help buffer systemic pH.
Carbonic anhydrase enzyme: This enzyme is found in two locations relevant to bicarbonate reabsorption: on the apical membrane of the PCT cells (facing the lumen) and within the PCT cells themselves. It catalyzes the rapid interconversion of carbon dioxide and water into carbonic acid, and vice versa.
The maintenance of a stable blood pH is one of the most tightly regulated physiological processes in the human body. Deviations from the narrow normal range can severely impair enzyme function and cellular processes, making the kidney’s role in acid-base homeostasis absolutely critical. A major part of this role is performed in the proximal convoluted tubule (PCT), where a significant portion of the filtered bicarbonate, the body’s primary extracellular buffer, is efficiently reclaimed. This diagram beautifully illustrates the elegant cellular mechanisms underlying bicarbonate reabsorption, emphasizing the pivotal role of the carbonic anhydrase enzyme.
The process of bicarbonate reabsorption in the PCT is a fascinating example of how the kidney indirectly reclaims an ion that cannot directly cross the luminal membrane. It begins with the active secretion of hydrogen ions (H+) from the PCT cell into the tubular lumen, primarily via Na+/H+ exchangers. In the lumen, this secreted H+ combines with filtered bicarbonate (HCO3-) to form carbonic acid (H2CO3). This reaction is rapidly catalyzed by carbonic anhydrase located on the luminal surface of the PCT cells. The carbonic acid then quickly dissociates into water (H2O) and carbon dioxide (CO2).
Both water and carbon dioxide are lipid-soluble and can readily diffuse across the apical membrane into the PCT cell. Once inside the cell, the reverse reaction occurs: CO2 and H2O, again catalyzed by intracellular carbonic anhydrase, combine to re-form carbonic acid. This intracellular H2CO3 then dissociates into H+ and HCO3-. The newly generated H+ is then secreted back into the tubular lumen (perpetuating the cycle), while the re-formed bicarbonate (HCO3-) is transported across the basolateral membrane of the PCT cell and into the interstitial fluid, eventually entering the bloodstream. This intricate mechanism effectively salvages filtered bicarbonate, preventing its loss in the urine and thereby preventing metabolic acidosis.
Dysregulation of bicarbonate reabsorption in the proximal convoluted tubule can lead to significant acid-base disturbances. Proximal renal tubular acidosis (pRTA) is a condition characterized by impaired bicarbonate reabsorption in the PCT, leading to excessive bicarbonate loss in the urine and systemic metabolic acidosis. This can be caused by genetic defects in the transporters or enzymes involved, or by certain drugs. Similarly, inhibition of carbonic anhydrase by drugs (e.g., acetazolamide) can decrease bicarbonate reabsorption, leading to metabolic acidosis and an increase in urinary pH. Understanding the precise steps and enzymatic actions involved in this process is paramount for diagnosing and treating these complex acid-base disorders, ensuring the body’s internal pH remains within a healthy range.

