The efficient movement of water across cell membranes is fundamental to virtually all physiological processes, from maintaining cell volume to urine concentration in the kidneys. This article delves into the intricate structure and function of aquaporin water channels, transmembrane proteins that selectively permit rapid water passage while preventing electrolyte leakage. Understanding aquaporins is crucial for comprehending cellular hydration, fluid balance, and the pathophysiology of various water-related disorders.
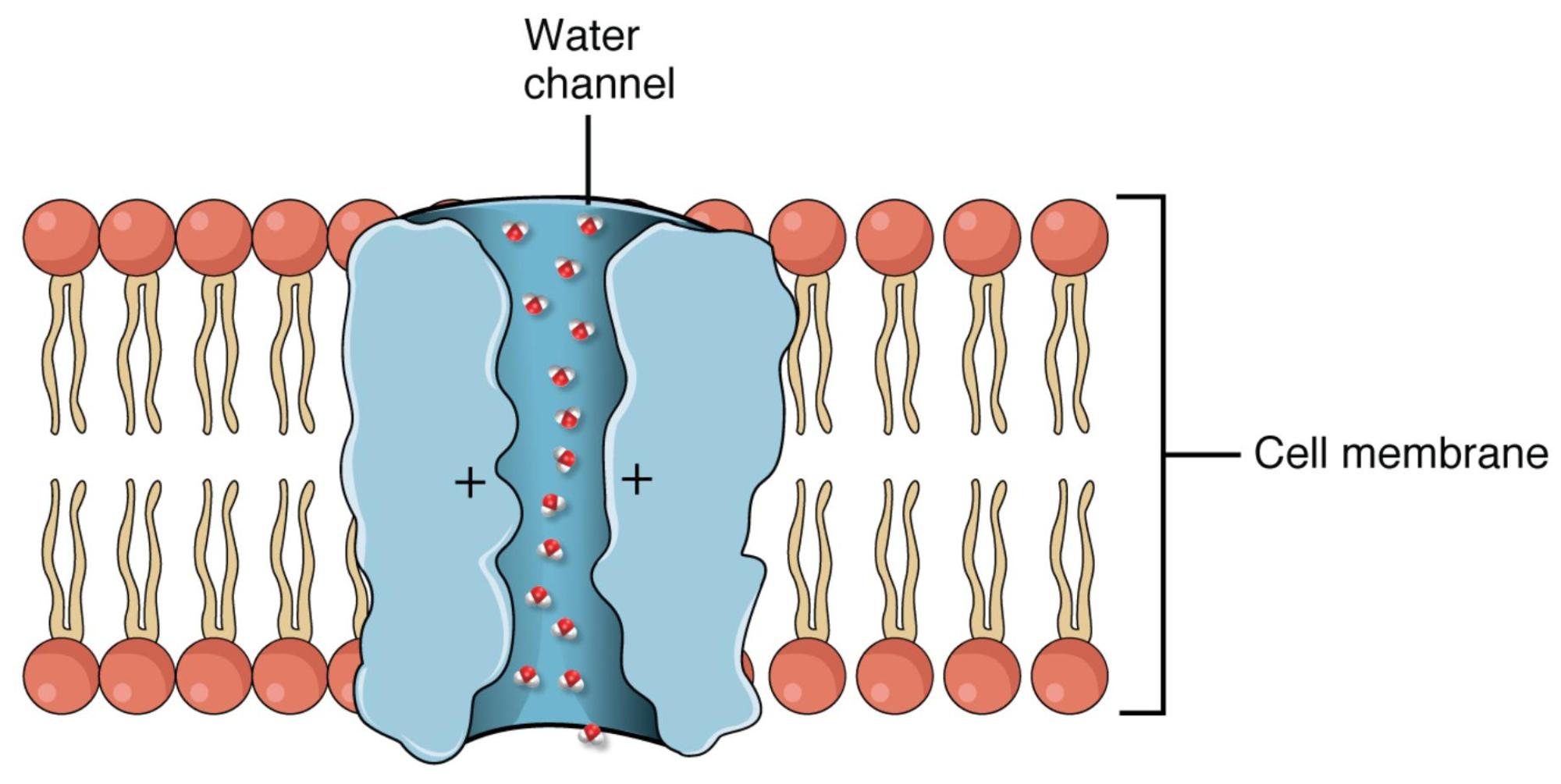
Water channel: This refers to the aquaporin protein embedded within the cell membrane, forming a selective pore specifically for water molecules. It allows for rapid, passive diffusion of water down its osmotic gradient.
Cell membrane: This is the outer boundary of an animal cell, composed of a phospholipid bilayer with embedded proteins. It acts as a selectively permeable barrier, controlling the movement of substances into and out of the cell.
Water is the most abundant molecule in the human body, and its precise movement across cellular barriers is critical for life. While water can slowly diffuse directly through the lipid bilayer of a cell membrane, this passive movement is often insufficient for organs that require rapid, bulk flow of water, such as the kidneys. This is where aquaporins come into play – specialized integral membrane proteins that serve as dedicated conduits for water molecules. The image clearly illustrates an aquaporin embedded within the cell membrane, acting as a selective gateway for water.
Aquaporins are a family of transmembrane proteins that form narrow channels, facilitating the rapid movement of water molecules across cell membranes via osmosis. These channels are highly selective, allowing water to pass through billions of molecules per second while completely excluding ions and other solutes. This selectivity is achieved through a unique internal architecture: the channel lumen is lined with specific amino acid residues that form a “water pore” perfectly sized for water molecules. Crucially, the presence of positive charges within the channel, as indicated in the diagram, creates an electrostatic barrier that repels charged ions and prevents the leakage of electrolytes, ensuring that water transport is uncoupled from ion movement. This exquisite design allows cells to regulate their water content and maintain osmotic balance without disrupting electrochemical gradients.
The physiological importance of aquaporins cannot be overstated. They are found in virtually all living organisms and are particularly abundant in tissues involved in fluid transport. In humans, different types of aquaporins (e.g., AQP1, AQP2, AQP3) are expressed in specific locations:
- Kidneys: Aquaporins, especially AQP1 in the proximal tubules and AQP2 in the collecting ducts, are vital for concentrating urine and maintaining body water balance. The insertion of AQP2 into the collecting duct membrane is regulated by antidiuretic hormone (ADH), allowing the kidney to adjust water reabsorption according to hydration status.
- Brain: Aquaporins play a role in cerebrospinal fluid production and brain water homeostasis.
- Gastrointestinal Tract: They facilitate water absorption in the intestines.
Dysfunction of aquaporins is implicated in a range of human diseases. For example, mutations in aquaporin genes can lead to conditions like nephrogenic diabetes insipidus, where the kidneys are unable to respond to ADH and reabsorb sufficient water, resulting in excessive urine production and severe dehydration. Conversely, increased expression or activity of certain aquaporins has been linked to conditions involving pathological fluid accumulation, such as cerebral edema or tumor growth. Research into aquaporin structure and regulation continues to offer promising avenues for developing new therapeutic strategies to manage disorders of fluid balance, highlighting the profound impact of these microscopic water channels on macroscopic human health.

