Explore the intricate biochemical processes of the Krebs cycle, also known as the citric acid cycle, and its pivotal role in cellular respiration. This essential metabolic pathway converts pyruvate into acetyl CoA, generating vital energy molecules like NADH, FADH2, and ATP that power our bodies.
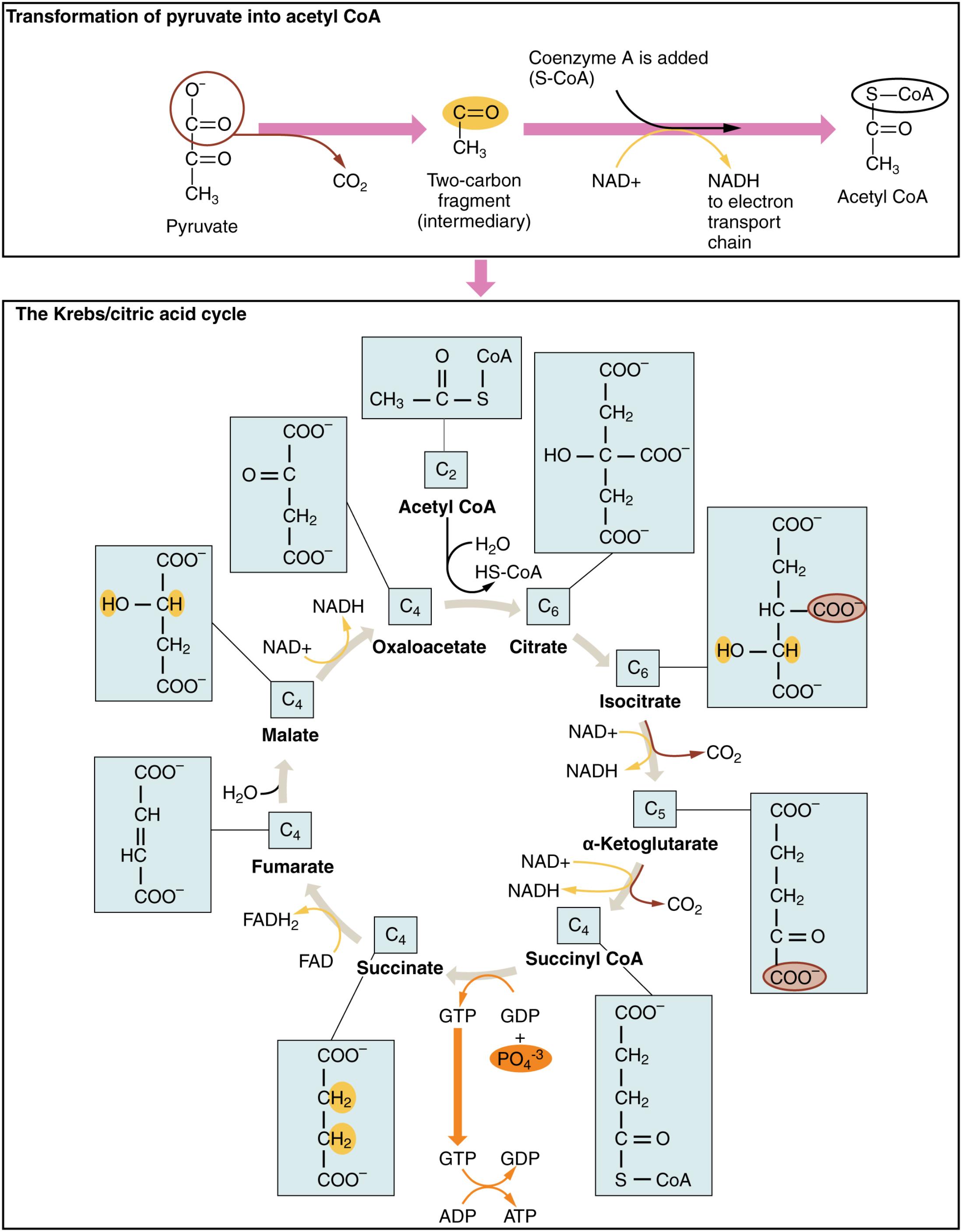
Understanding the Krebs Cycle Diagram
Pyruvate: This three-carbon molecule is the end product of glycolysis, the initial stage of glucose metabolism. It serves as the primary input for further energy extraction within the mitochondria, marking a crucial transition point in cellular respiration.
Coenzyme A: A vital coenzyme derived from pantothenic acid (vitamin B5), it plays a crucial role in the metabolism of carbohydrates, fats, and proteins. Coenzyme A facilitates the formation of acetyl CoA, enabling its entry into the Krebs cycle.
Two-carbon fragment (intermediary): This represents the acetyl group, a two-carbon unit derived from pyruvate after decarboxylation. This fragment is the core component that combines with coenzyme A to form acetyl CoA, making it ready for the next stage of energy production.
NAD+: Nicotinamide adenine dinucleotide, in its oxidized form, acts as a critical electron acceptor in various metabolic reactions. During the conversion of pyruvate to acetyl CoA and within the Krebs cycle itself, NAD+ is reduced to NADH, carrying high-energy electrons to the electron transport chain.
NADH to electron transport chain: NADH, the reduced form of NAD+, carries high-energy electrons that are vital for ATP production. These electrons are transferred to the electron transport chain, where their energy is harnessed to pump protons and drive the synthesis of the majority of cellular ATP.
Acetyl CoA: Acetyl coenzyme A is a central molecule in metabolism, connecting glycolysis, fatty acid oxidation, and amino acid catabolism to the Krebs cycle. Its entry into the cycle marks the commitment of carbon atoms for complete oxidation and significant energy generation.
Oxaloacetate: This four-carbon molecule is the starting and regenerating compound of the Krebs cycle. It condenses with acetyl CoA to form citrate, initiating the cyclical series of reactions that lead to the production of energy carriers.
Citrate: A six-carbon tricarboxylic acid formed by the condensation of acetyl CoA and oxaloacetate, citrate is the first intermediate of the Krebs cycle. Its formation signifies the beginning of the oxidative breakdown of carbon atoms within the cycle.
Isocitrate: This isomer of citrate is formed through an isomerization reaction. It is subsequently decarboxylated and oxidized, leading to the production of alpha-ketoglutarate and the first release of CO2 and NADH in the cycle.
α-Ketoglutarate: A five-carbon alpha-keto acid, alpha-ketoglutarate is a key intermediate in both the Krebs cycle and amino acid metabolism. Its conversion to succinyl CoA is another oxidative decarboxylation step, yielding more CO2 and NADH.
Succinyl CoA: This four-carbon molecule with an attached coenzyme A is formed from alpha-ketoglutarate. The cleavage of its high-energy thioester bond drives the phosphorylation of GDP to GTP, an example of substrate-level phosphorylation.
GTP + PO4 3-: Guanosine triphosphate (GTP) is an energy-rich molecule similar to ATP. The phosphate group (PO4 3-) released during the conversion of succinyl CoA to succinate is used to synthesize GTP from GDP, which can then be converted to ATP.
GDP: Guanosine diphosphate, when phosphorylated, forms GTP. This reaction is crucial for energy transfer within the cell and is directly linked to the activity of the Krebs cycle.
ATP: Adenosine triphosphate is the primary energy currency of the cell, essential for powering almost all cellular processes. While some ATP is directly produced in the Krebs cycle (via GTP), the majority is generated through oxidative phosphorylation driven by NADH and FADH2.
Succinate: A four-carbon dicarboxylic acid, succinate is formed after the removal of coenzyme A from succinyl CoA. Its oxidation to fumarate produces FADH2, another critical electron carrier for the electron transport chain.
FADH2: Flavin adenine dinucleotide, in its reduced form, is another vital electron carrier in cellular respiration. FADH2 delivers its high-energy electrons to the electron transport chain at a slightly lower energy level than NADH, contributing to ATP synthesis.
Fumarate: This four-carbon dicarboxylic acid is formed from the oxidation of succinate. Its hydration to malate is a key step in regenerating oxaloacetate and continuing the cycle.
Malate: A four-carbon dicarboxylic acid, malate is formed from fumarate through the addition of water. Its subsequent oxidation to oxaloacetate completes the cycle and produces the final molecule of NADH within the Krebs cycle.
The Krebs cycle, also known as the citric acid cycle or tricarboxylic acid (TCA) cycle, stands as a central pillar of cellular respiration, the intricate process by which organisms convert nutrients into energy. This metabolic pathway is not merely a series of reactions but a finely tuned engine operating within the mitochondria of eukaryotic cells. Its primary function is to complete the oxidation of carbohydrates, fats, and proteins, transforming their chemical energy into a usable form for the cell. Without this cycle, the vast majority of ATP, the universal energy currency, could not be generated efficiently, making it indispensable for life.
At its core, the Krebs cycle systematically processes acetyl CoA, a two-carbon molecule derived from the breakdown of glucose (via pyruvate), fatty acids, and certain amino acids. This molecule enters the cycle by condensing with a four-carbon compound, oxaloacetate, to form citrate. Through a series of eight enzyme-catalyzed reactions, the citrate molecule is progressively oxidized, releasing carbon dioxide and generating reduced coenzymes: NADH and FADH2. These high-energy electron carriers are then shuttled to the electron transport chain, where their energy is ultimately used to drive the synthesis of ATP through oxidative phosphorylation.
The significance of the Krebs cycle extends beyond energy production. It serves as a metabolic hub, providing precursors for various biosynthetic pathways, including the synthesis of amino acids, fatty acids, and heme. This dual role in both catabolism (breaking down molecules) and anabolism (building up molecules) underscores its foundational importance in maintaining cellular homeostasis. The cycle’s intricate regulation ensures that energy production is balanced with the cell’s metabolic demands, adapting to varying nutritional states and physiological conditions.
- The Krebs cycle is a metabolic pathway that occurs in the mitochondria.
- It generates ATP, NADH, and FADH2.
- It is crucial for both energy production and biosynthesis.
- It processes acetyl CoA derived from carbohydrates, fats, and proteins.
The Mechanism of Energy Production
The journey of energy extraction begins with glucose, which is first broken down into two molecules of pyruvate through glycolysis in the cytoplasm. These pyruvate molecules then enter the mitochondria, where they undergo an oxidative decarboxylation reaction, converting each pyruvate into a two-carbon acetyl CoA molecule, while simultaneously reducing NAD+ to NADH and releasing carbon dioxide. This acetyl CoA is the crucial entry point into the Krebs cycle, linking glycolysis to the subsequent stages of aerobic respiration. The formation of acetyl CoA is a highly regulated step, often considered the commitment step for glucose oxidation.
Once inside the cycle, acetyl CoA combines with oxaloacetate to form citrate, initiating a series of transformations that ultimately regenerate oxaloacetate, allowing the cycle to continue. Throughout these reactions, carbon atoms are fully oxidized, leading to the release of carbon dioxide as a waste product. More importantly, the cycle captures chemical energy in the form of reduced coenzymes: three molecules of NADH and one molecule of FADH2 for each turn of the cycle. Additionally, one molecule of ATP (or GTP, which can be readily converted to ATP) is generated directly through substrate-level phosphorylation, showcasing the cycle’s multifaceted energy yield.
The high-energy electrons carried by NADH and FADH2 are not immediately used to generate ATP within the Krebs cycle itself. Instead, these electron carriers act as crucial intermediaries, transporting their captured energy to the electron transport chain, located on the inner mitochondrial membrane. Here, a series of protein complexes facilitate the stepwise transfer of electrons, harnessing their energy to pump protons across the membrane, creating an electrochemical gradient. This proton gradient then drives the synthesis of the vast majority of cellular ATP through a process known as chemiosmosis, illustrating the interconnectedness of cellular respiration’s stages.
Concluding Thoughts on Cellular Energy
The Krebs cycle represents a masterpiece of biological engineering, a cyclical pathway that efficiently extracts energy from our diet and orchestrates the synthesis of vital building blocks. Its continuous operation is fundamental to sustaining life, underpinning everything from muscle contraction to neuronal activity. Understanding this intricate process provides profound insights into cellular metabolism, offering a foundation for exploring metabolic disorders and developing therapeutic strategies aimed at optimizing energy production and overall health.

