The complement system is a crucial component of the immune response, enhancing the body’s ability to fight infections through a series of protein interactions. Activated primarily during adaptive immunity, this cascade amplifies the effects of antibodies by marking pathogens for destruction, with the classical pathway initiated when C1 binds to antigen-antibody complexes. This illustration provides a detailed look at the complement cascade’s stages and its vital role in bridging innate and adaptive immunity.
Labeled Components of the Complement Cascade
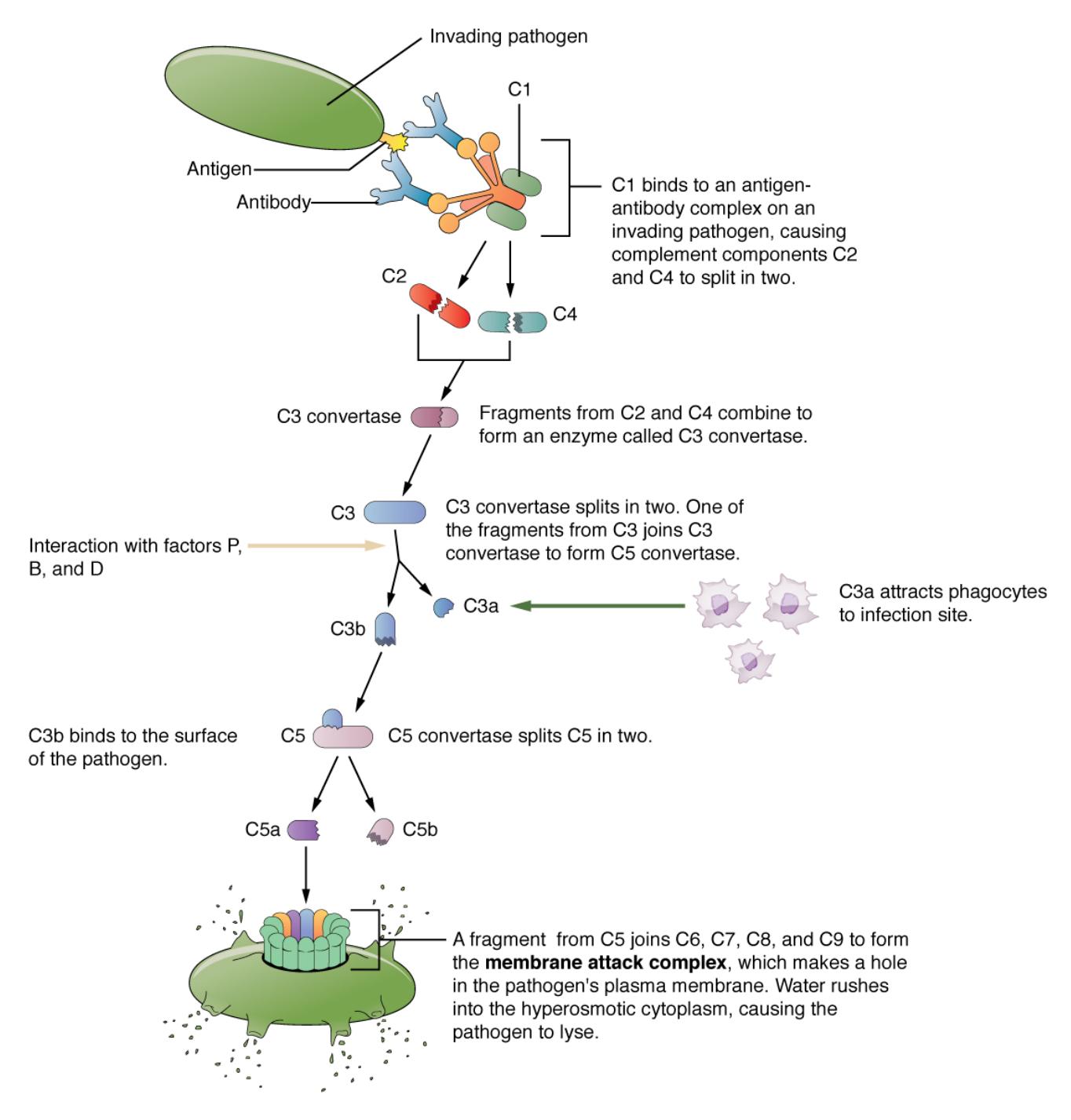
C1: This initial complement protein binds to antigen-antibody complexes, triggering the classical pathway. It activates subsequent components, setting the cascade in motion.
C2: Activated by C1, C2 splits into C2a and C2b, with C2a contributing to the formation of the C3 convertase. This step is essential for amplifying the immune response.
C3: A central protein in the cascade, C3 is cleaved into C3a and C3b by C3 convertase. C3b opsonizes pathogens, while C3a promotes inflammation.
C3 convertase: Formed by the combination of C4b and C2a, this enzyme cleaves C3 into active fragments. It is a pivotal step that drives the cascade forward.
C4: Activated by C1, C4 splits into C4a and C4b, with C4b binding to the target surface. This binding facilitates the assembly of C3 convertase.
C5: Cleaved by C5 convertase into C5a and C5b, C5 initiates the terminal pathway. C5b triggers the formation of the membrane attack complex.
C5 convertase: This enzyme, formed by C3b and additional components, cleaves C5 to advance the cascade. It marks the transition to the lytic phase of complement action.
C6: Combining with C5b, C6 helps assemble the membrane attack complex on pathogen surfaces. It stabilizes the structure for effective cell lysis.
C7: This protein joins C5b-6 to further build the membrane attack complex. It enhances the complex’s ability to insert into pathogen membranes.
C8: Integrating with C5b-7, C8 facilitates the polymerization of C9. It creates a channel in the pathogen’s membrane, initiating lysis.
C9: This final component polymerizes to form the membrane attack complex’s pore. It disrupts the pathogen’s membrane, leading to cell death.
Antigen: A foreign molecule that triggers an immune response, recognized by antibodies. Its binding to antibodies activates the classical complement pathway.
Antibody: Produced by B cells, antibodies bind to antigens, marking pathogens for complement activation. This interaction initiates the C1-mediated cascade.
Pathogen: The target of the complement system, such as bacteria or viruses, is coated with complement proteins. This coating enhances immune clearance.
Opsonization: The process where C3b coats pathogens, making them more recognizable to phagocytes. It significantly boosts the efficiency of pathogen removal.
Inflammation: Triggered by C3a and C5a, this response recruits immune cells to the infection site. It amplifies the overall immune reaction.
Membrane attack complex (MAC): Formed by C5b through C9, this structure punctures pathogen membranes. It leads to osmotic lysis and pathogen destruction.
Phagocyte: Immune cells like macrophages and neutrophils engulf opsonized pathogens. They are activated by complement fragments to enhance clearance.
Overview of the Complement Cascade
The complement system operates through a series of enzymatic reactions, enhancing immune defense mechanisms.
- C1 binds to antigen-antibody complexes, initiating the classical pathway during adaptive immunity.
- C4 and C2 activation forms C3 convertase, a critical enzyme in the cascade.
- C3 cleavage produces C3b for opsonization and C3a for inflammation.
- C5 convertase drives the formation of the membrane attack complex.
- C6 through C9 assemble to lyse pathogen cells, completing the terminal pathway.
- Phagocytes recognize opsonized pathogens, aiding in their destruction.
This illustration highlights the cascade’s progression from activation to pathogen elimination.
The Classical Pathway in Action
The classical pathway is a key mechanism linking adaptive immunity to complement function.
- Antibodies bind to antigens on pathogens, providing a target for C1 activation.
- C1 cleaves C4 into C4a and C4b, with C4b binding to the pathogen surface.
- C2 is split by C1, and C2a combines with C4b to form C3 convertase.
- C3 convertase amplifies the response by cleaving C3 into C3a and C3b.
- C3b enhances opsonization, while C3a triggers inflammation.
- This pathway ensures a targeted response to specific pathogens.
This process underscores the complement system’s role in adaptive immunity.
Functions of Complement Proteins
Each complement component contributes uniquely to immune defense and regulation.
- C5a and C3a act as anaphylatoxins, promoting inflammation by recruiting phagocytes.
- Opsonization by C3b marks pathogens, increasing phagocyte efficiency.
- The membrane attack complex lyses gram-negative bacteria and other pathogens.
- C1 to C4 activation provides specificity, linking to antibody recognition.
- C5 to C9 execution ensures direct pathogen destruction.
- Phagocytes clear debris, preventing tissue damage from inflammation.
This multifaceted action enhances overall immune efficacy.
Clinical Significance of the Complement Cascade
Understanding the complement system aids in diagnosing and treating immune disorders.
- Deficiencies in C3 or C5 can lead to recurrent infections, reducing opsonization.
- Overactive complement activity may contribute to autoimmune diseases like lupus.
- Membrane attack complex dysfunction can impair bacterial clearance.
- C1 inhibitor deficiency causes hereditary angioedema, linked to excessive inflammation.
- Phagocyte response issues may indicate complement pathway defects.
- Monitoring C3 levels helps assess immune competence in chronic conditions.
This knowledge is essential for tailoring therapeutic approaches.
The complement cascade, as depicted in this illustration, is a remarkable example of immune coordination, bridging adaptive and innate responses. By amplifying antibody effects and directly attacking pathogens, it plays an indispensable role in maintaining health, offering a fascinating area of study for anyone interested in immunology.

