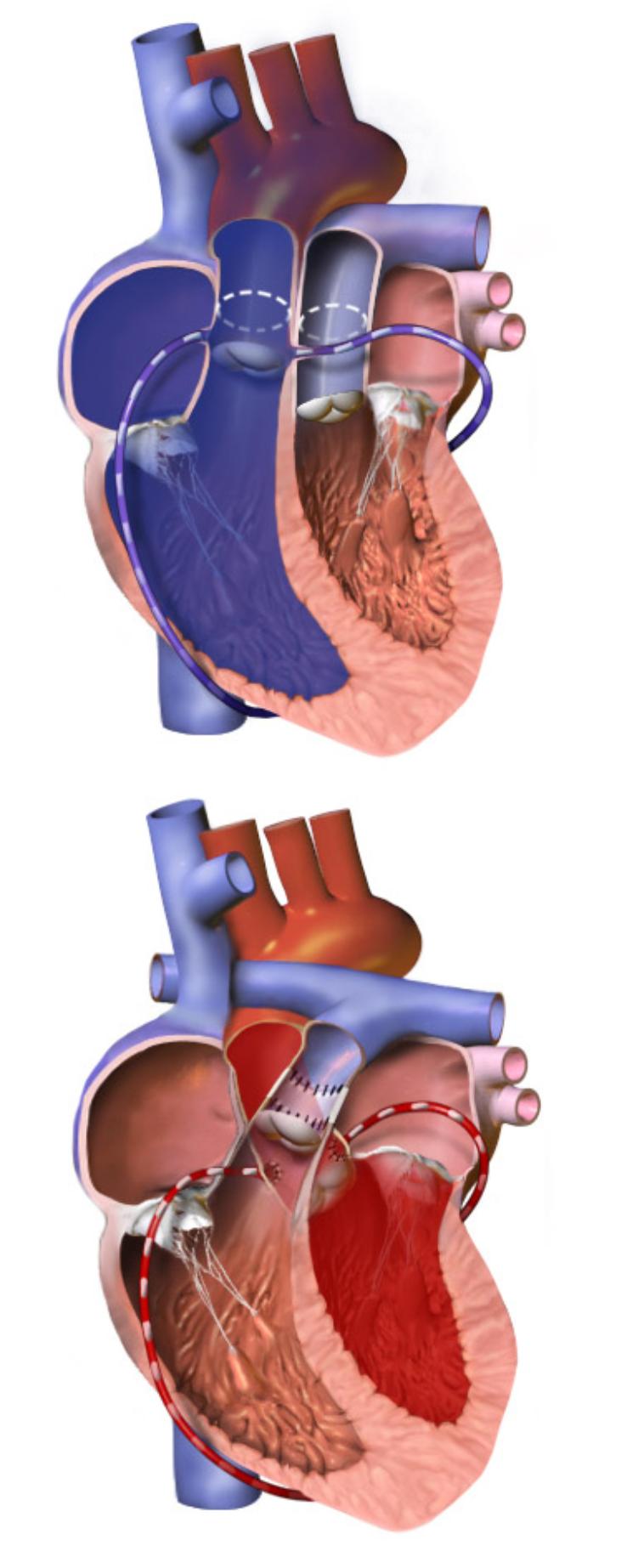
This diagram provides a clear visual explanation of the Arterial Switch Operation (ASO), a complex but life-saving surgical procedure used to correct transposition of the great arteries (TGA). The top panel illustrates the heart’s anatomy before the operation, highlighting the abnormal connections of the great arteries. The bottom panel demonstrates the corrected anatomy post-surgery, showcasing how the arteries are reconnected to ensure proper blood flow. This detailed visual guide is crucial for understanding how this intricate surgery restores normal cardiovascular circulation in affected infants.
Introduction to the Arterial Switch Operation
Transposition of the Great Arteries (TGA) is a critical congenital heart defect in which the two major arteries leaving the heart—the aorta and the pulmonary artery—are switched. In a normal heart, the aorta carries oxygenated blood from the left ventricle to the body, and the pulmonary artery carries deoxygenated blood from the right ventricle to the lungs. In TGA, these connections are reversed: the aorta arises from the right ventricle, carrying deoxygenated blood to the body, while the pulmonary artery arises from the left ventricle, sending oxygenated blood back to the lungs. This creates two parallel circulatory systems, which is incompatible with life unless there are other openings (like a patent foramen ovale or ductus arteriosus) that allow some mixing of blood. The Arterial Switch Operation (ASO) is the gold standard surgical procedure to correct this life-threatening condition.
The diagram effectively illustrates the profound anatomical correction achieved by the ASO. Before the switch, deoxygenated blood circulates endlessly through the body, while oxygenated blood is trapped in a loop to the lungs. This leads to severe cyanosis (a bluish discoloration of the skin due to lack of oxygen) shortly after birth. The ASO involves surgically reconnecting the aorta to the left ventricle and the pulmonary artery to the right ventricle, along with re-implanting the coronary arteries. This restoration of normal anatomical connections allows for proper oxygenation of the blood and its efficient delivery to the body.
Performing the ASO typically occurs within the first few weeks of an infant’s life, often within the first two weeks, as early intervention is crucial for survival and optimal outcomes. The complexity of the surgery requires highly specialized surgical teams and advanced neonatal intensive care. Understanding the mechanics of this operation, as depicted, is essential for comprehending how modern pediatric cardiac surgery addresses severe congenital heart anomalies.
Key characteristics of Transposition of the Great Arteries (TGA) and reasons for ASO include:
- Cyanosis: A primary symptom due to inadequate oxygenation of systemic blood.
- Parallel Circulations: Deoxygenated blood circulates to the body, and oxygenated blood circulates to the lungs.
- Ductal-Dependent Lesion: Survival often depends on a patent ductus arteriosus (PDA) or atrial septal defect (ASD) to allow blood mixing.
- Pulmonary Hypertension: Can develop if the left ventricle maintains systemic pressures for too long.
- Improved Long-Term Outcomes: ASO offers better long-term ventricular function and quality of life compared to older palliative procedures.
The timely diagnosis and surgical correction are paramount for infants with TGA.
The Anatomy of TGA and the Need for ASO
In a healthy heart, the right atrium receives deoxygenated blood from the body and pumps it to the right ventricle. The right ventricle then pumps this blood into the pulmonary artery, which carries it to the lungs for oxygenation. Simultaneously, the left atrium receives oxygenated blood from the lungs and sends it to the left ventricle. The left ventricle, being the strongest chamber, then pumps this oxygenated blood into the aorta, which distributes it throughout the body.
In Transposition of the Great Arteries (TGA), this fundamental anatomical arrangement is reversed. The aorta originates from the right ventricle, and the pulmonary artery originates from the left ventricle. This means the right ventricle, which is normally accustomed to pumping against the low resistance of the pulmonary circulation, is now tasked with pumping deoxygenated blood against the high resistance of the systemic circulation. Conversely, the left ventricle, adapted for systemic pressures, is now pumping oxygenated blood into the low-pressure pulmonary circulation. This creates two entirely separate circulatory loops, as depicted in the top panel of the diagram. The body receives insufficient oxygen, leading to severe hypoxia and a life-threatening situation.
For immediate survival, infants with TGA often rely on existing fetal shunts—a patent foramen ovale (PFO) and/or a patent ductus arteriosus (PDA)—which allow some mixing of oxygenated and deoxygenated blood. These shunts provide a temporary lifeline, but they are typically insufficient for long-term survival. Often, a balloon atrial septostomy (Rashkind procedure) is performed shortly after birth to enlarge the atrial septal defect, allowing for better mixing of blood. However, the definitive treatment is the Arterial Switch Operation, which anatomically corrects the defect.
The Arterial Switch Operation: Surgical Steps
The Arterial Switch Operation is a complex open-heart surgery performed under cardiopulmonary bypass, meaning a heart-lung machine temporarily takes over the functions of the heart and lungs. The procedure, as visualized in the bottom panel of the diagram, primarily involves the following critical steps:
- Transection of the Great Arteries: Both the aorta and the pulmonary artery are cut just above their respective valves.
- Coronary Artery Transfer: This is one of the most challenging parts of the operation. The coronary arteries, which originate from the aorta and supply blood to the heart muscle itself, are carefully detached from the transposed aorta. They are then re-implanted onto the newly positioned (neo-)aorta, which will now arise from the left ventricle. Successful re-implantation is vital to ensure the heart muscle receives adequate blood supply.
- Aortic Re-implantation: The distal end of the aorta is then sewn to the proximal end of the original pulmonary artery stump, which is now connected to the left ventricle. This effectively re-establishes the aorta’s connection to the oxygenated blood supply.
- Pulmonary Artery Re-implantation: The distal end of the pulmonary artery is connected to the proximal end of the original aortic stump, which is now connected to the right ventricle. This allows the right ventricle to pump deoxygenated blood to the lungs.
- Reconstruction of the Pulmonary Artery: Because the pulmonary artery is often smaller after the switch and needs to accommodate the coronary arteries’ transfer sites, the area is reconstructed, often using patches, to ensure proper size and flow.
This intricate sequence of steps results in the physiological correction, allowing the left ventricle to pump oxygenated blood to the body and the right ventricle to pump deoxygenated blood to the lungs, mimicking normal circulation.
Post-Operative Care and Long-Term Outlook
Following an Arterial Switch Operation, infants require intensive post-operative care in a specialized neonatal or pediatric intensive care unit. This includes careful monitoring of cardiac function, respiratory status, fluid balance, and pain management. Complications can include issues related to coronary artery function, pulmonary artery stenosis at the reconstruction site, or residual ventricular dysfunction.
Despite the complexity, the ASO has dramatically improved the prognosis for infants with TGA. The long-term outlook for children who undergo this procedure is generally excellent, with high survival rates and good functional outcomes. Children typically lead active, normal lives. However, lifelong follow-up with a pediatric cardiologist is essential to monitor for potential late complications. These may include mild narrowing of the pulmonary arteries, issues with the coronary arteries, or ventricular dysfunction. Regular check-ups with echocardiograms, electrocardiograms, and sometimes MRI scans help ensure the continued health of the heart and prompt identification of any potential issues, allowing for timely intervention if needed.

