Explore the fundamental principles of partial and total gas pressures, crucial for understanding respiratory physiology and gas exchange in the body. This article explains how individual gas pressures contribute to the overall atmospheric pressure and influence the movement of oxygen and nitrogen, vital for medical applications.
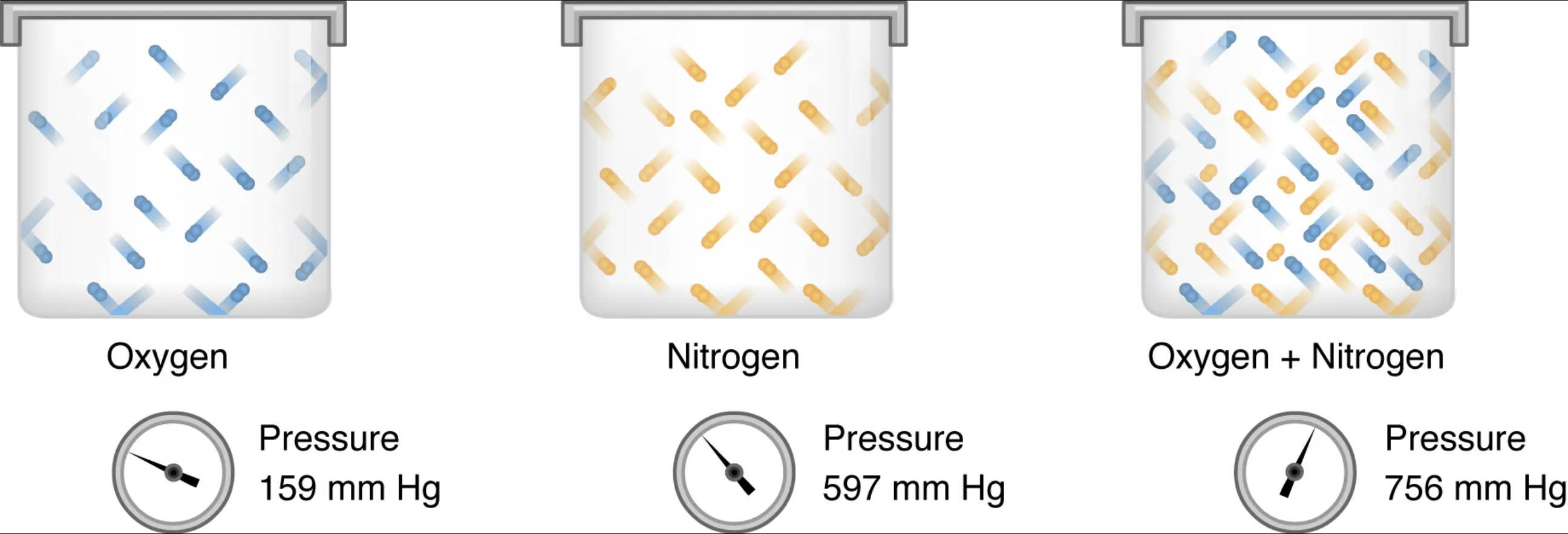
Oxygen: Represented by blue elongated shapes, this illustrates oxygen molecules exerting a specific pressure within a contained environment. Oxygen is a vital gas for human respiration, playing a key role in cellular metabolism and energy production.
Pressure 159 mm Hg (for Oxygen): This value indicates the partial pressure exerted by oxygen alone within its container. In atmospheric air at sea level, oxygen contributes significantly to the total pressure, facilitating its diffusion into the bloodstream.
Nitrogen: Represented by orange elongated shapes, this depicts nitrogen molecules, the most abundant gas in the Earth’s atmosphere. While largely inert in human physiology at normal pressures, nitrogen still contributes to the overall gas pressure within the lungs.
Pressure 597 mm Hg (for Nitrogen): This value shows the partial pressure exerted by nitrogen within its own container. Nitrogen’s high partial pressure in ambient air means it plays a critical role in determining the total atmospheric pressure.
Oxygen + Nitrogen: This image displays a mixture of both oxygen (blue) and nitrogen (orange) molecules within a single container. This visualizes a gas mixture, similar to the air we breathe, where multiple gases coexist and contribute to the total pressure.
Pressure 756 mm Hg (for Oxygen + Nitrogen): This value represents the total pressure exerted by the mixture of oxygen and nitrogen gases. According to Dalton’s Law, this total pressure is the sum of the individual partial pressures of each gas in the mixture.
The Invisible Forces: Understanding Gas Pressures in Medicine
The air we breathe is not a single entity but a complex mixture of various gases, primarily nitrogen, oxygen, and trace amounts of others. Each of these gases exerts its own individual force, known as partial pressure, which collectively adds up to the total atmospheric pressure. This fundamental principle of gas dynamics is not merely an academic concept but a cornerstone of respiratory physiology, crucial for understanding how our bodies efficiently extract oxygen and expel carbon dioxide.
In medical contexts, comprehending partial and total gas pressures is vital for explaining phenomena such as gas exchange in the lungs, oxygen delivery to tissues, and the physiological responses to changes in atmospheric pressure, as experienced by divers or high-altitude climbers. Deviations from normal gas pressures in the body can indicate serious medical conditions, making these measurements essential diagnostic tools.
Key principles illustrated:
- Each gas in a mixture exerts an independent partial pressure.
- The sum of these partial pressures equals the total pressure of the gas mixture.
- These principles are governed by Dalton’s Law of Partial Pressures.
This understanding underpins critical medical interventions and the study of respiratory diseases.
Dalton’s Law: The Sum of the Parts
Dalton’s Law of Partial Pressures states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This law is foundational to respiratory physiology and helps explain the movement of gases within the lungs and across the alveolar-capillary membrane. The atmosphere at sea level, for instance, has a total pressure of approximately 760 mm Hg. This pressure is the aggregate of the partial pressures of its constituent gases, primarily nitrogen, oxygen, carbon dioxide, and water vapor.
Oxygen: The Breath of Life
Oxygen constitutes about 21% of the atmospheric air. Its partial pressure, denoted as PO2, is approximately 159 mm Hg at sea level (0.21 x 760 mm Hg). This partial pressure is a critical driving force for oxygen to diffuse from the atmosphere into the alveoli of the lungs, and subsequently into the bloodstream. A sufficient PO2 gradient between the alveoli and the pulmonary capillaries is essential for adequate oxygenation of the blood. Conditions like hypoxemia, where blood oxygen levels are low, often correlate with reduced partial pressures of oxygen, highlighting its medical significance.
Nitrogen: The Silent Majority
Nitrogen is the most abundant gas in the atmosphere, making up about 78% of the air we breathe. Its partial pressure, PN2, is approximately 597 mm Hg at sea level (0.78 x 760 mm Hg). While nitrogen is largely inert under normal atmospheric pressures and does not actively participate in gas exchange, its high partial pressure contributes significantly to the total pressure exerted by the air. In specialized medical contexts, such as hyperbaric medicine or diving, nitrogen’s behavior under increased pressure becomes highly relevant, particularly concerning decompression sickness (the bends), where dissolved nitrogen can form bubbles in tissues and blood if pressure is reduced too quickly.
The Interplay in Gas Mixtures
When oxygen and nitrogen are mixed, as shown in the combined illustration, their individual partial pressures add up to the total pressure of the mixture. For example, if oxygen exerts a pressure of 159 mm Hg and nitrogen exerts 597 mm Hg, the total pressure of the gas mixture will be 159 mm Hg + 597 mm Hg = 756 mm Hg (allowing for slight variations due to other trace gases and water vapor in real atmospheric air). This additive property is fundamental to understanding how gases behave in the respiratory system. The partial pressure gradient of oxygen from the alveoli (around 104 mm Hg) to the pulmonary capillaries (around 40 mm Hg) drives oxygen into the blood. Similarly, the partial pressure gradient of carbon dioxide (PCO2) facilitates its movement out of the blood and into the alveoli for exhalation. These gradients are crucial for maintaining efficient gas exchange and overall physiological homeostasis.
Conclusion
The concepts of partial and total gas pressures, as defined by Dalton’s Law, are foundational to understanding respiratory physiology and have profound implications in various medical fields. From the critical exchange of oxygen and carbon dioxide in the lungs to the complexities of hyperbaric medicine, the precise measurement and interpretation of these pressures are indispensable. A clear grasp of how individual gases contribute to the overall atmospheric pressure, and how these forces drive gas movement within the body, empowers healthcare professionals to better diagnose and manage respiratory and related conditions, ensuring optimal patient care.

