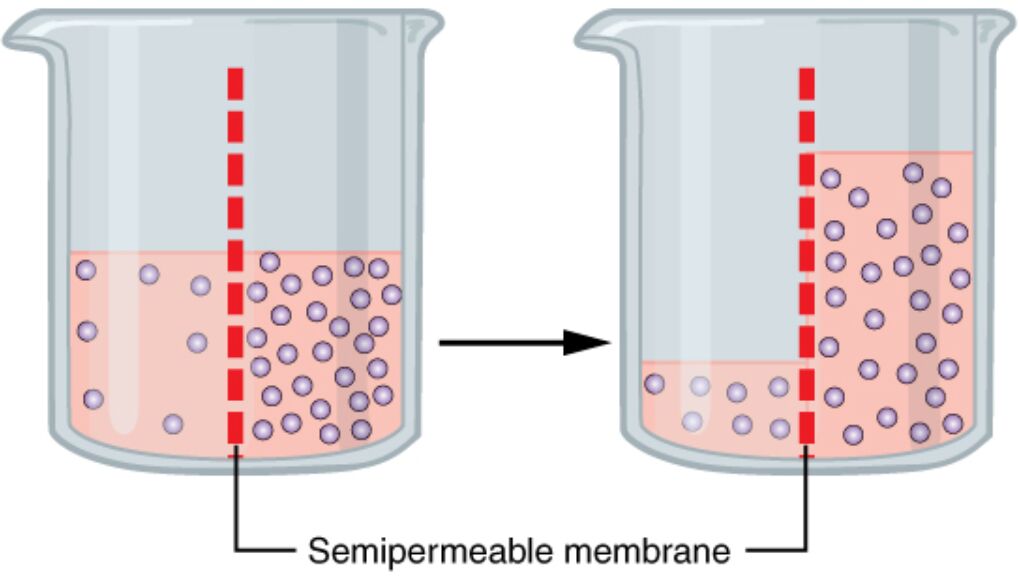
Osmosis is a fundamental biological process that governs the movement of water across a semipermeable membrane, playing a crucial role in maintaining cellular balance. This passive transport mechanism allows water to diffuse down its concentration gradient, from areas of lower solute concentration to higher solute concentration, as depicted in the beaker setup where the right side is hypertonic. In this article, we will explore the mechanics of osmosis, the role of the semipermeable membrane, and its significance in cellular and physiological functions.
Labeled Components of Osmosis
Semipermeable Membrane
The semipermeable membrane acts as a selective barrier, permitting the passage of water molecules while restricting solutes. In the diagram, it divides the beaker into two compartments, facilitating the movement of water from the left (lower solute concentration) to the right (higher solute concentration).
Hypertonic Solution
The hypertonic solution, shown on the right side of the left beaker, has a higher solute concentration compared to the left side. This concentration difference drives water to move across the semipermeable membrane to equalize the water concentration on both sides.
The Anatomy and Physical Properties of Osmosis
Structure of the Semipermeable Membrane
The semipermeable membrane is a key component in osmosis, designed to regulate molecular passage. Here’s a closer look:
- The semipermeable membrane is typically composed of a lipid bilayer with embedded proteins, allowing water to pass while blocking larger solutes like sugars or salts.
- Its selective permeability is due to the hydrophobic nature of the lipid bilayer, which restricts the movement of polar or charged molecules.
- Pores formed by proteins, such as aquaporins, enhance the membrane’s ability to facilitate water transport during osmosis.
- The membrane’s integrity ensures that only water moves, maintaining the solute concentration gradient that drives the process.
Physical Principles of Osmosis
Osmosis operates based on well-defined physical principles. Key aspects include:
- Water moves down its concentration gradient, from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration), as seen in the hypertonic solution.
- The process is passive, requiring no energy input from the cell, as it relies solely on the osmotic pressure gradient.
- The rate of osmosis depends on factors like the surface area of the membrane, the steepness of the concentration gradient, and the presence of aquaporins.
- Equilibrium is achieved when the water concentration on both sides of the membrane is equal, halting net water movement.
Functional Roles of Osmosis in Cellular and Physiological Processes
Water Balance in Cells
Osmosis is essential for regulating water balance within cells. This process supports:
- In a hypertonic environment, water exits the cell, causing it to shrink, a phenomenon known as plasmolysis in plant cells.
- In a hypotonic environment, water enters the cell, potentially causing it to swell or even burst if the influx is excessive, as seen in red blood cells.
- Plant cells rely on osmosis to maintain turgor pressure, which keeps them rigid and supports their structure.
- The process ensures that cells maintain an optimal internal environment, supporting metabolic activities and preventing cellular damage.
Osmosis in Physiological Systems
Osmosis plays a critical role in various physiological systems. Here’s how:
- In the kidneys, osmosis facilitates the reabsorption of water in the nephron, concentrating urine and maintaining the body’s fluid balance.
- In the intestines, water is absorbed via osmosis during digestion, ensuring proper hydration and nutrient uptake into the bloodstream.
- Osmosis regulates blood plasma osmolarity, which is crucial for maintaining blood pressure and preventing conditions like dehydration or edema.
- The process supports the movement of water into and out of capillaries, balancing fluid levels between blood and interstitial spaces.
Impact on Cellular Homeostasis
Osmosis is vital for maintaining cellular homeostasis. Key points include:
- The movement of water across the semipermeable membrane helps balance intracellular and extracellular fluid levels, preventing osmotic stress.
- It ensures that cells in isotonic environments maintain their shape and function without excessive shrinking or swelling.
- Osmosis supports the transport of nutrients and waste by maintaining proper fluid dynamics within the cell.
- The process is critical for cells lacking rigid walls, like animal cells, to avoid lysis or crenation due to osmotic imbalances.
Conclusion
Osmosis, the diffusion of water through a semipermeable membrane, is a cornerstone of cellular and physiological function, ensuring the proper distribution of water across membranes. The semipermeable membrane and the dynamics of hypertonic solutions highlight the elegance of this passive process in maintaining equilibrium and supporting life. By understanding osmosis, we gain insight into the intricate mechanisms that regulate water balance, cellular integrity, and overall physiological health, underscoring its importance in biological systems.

