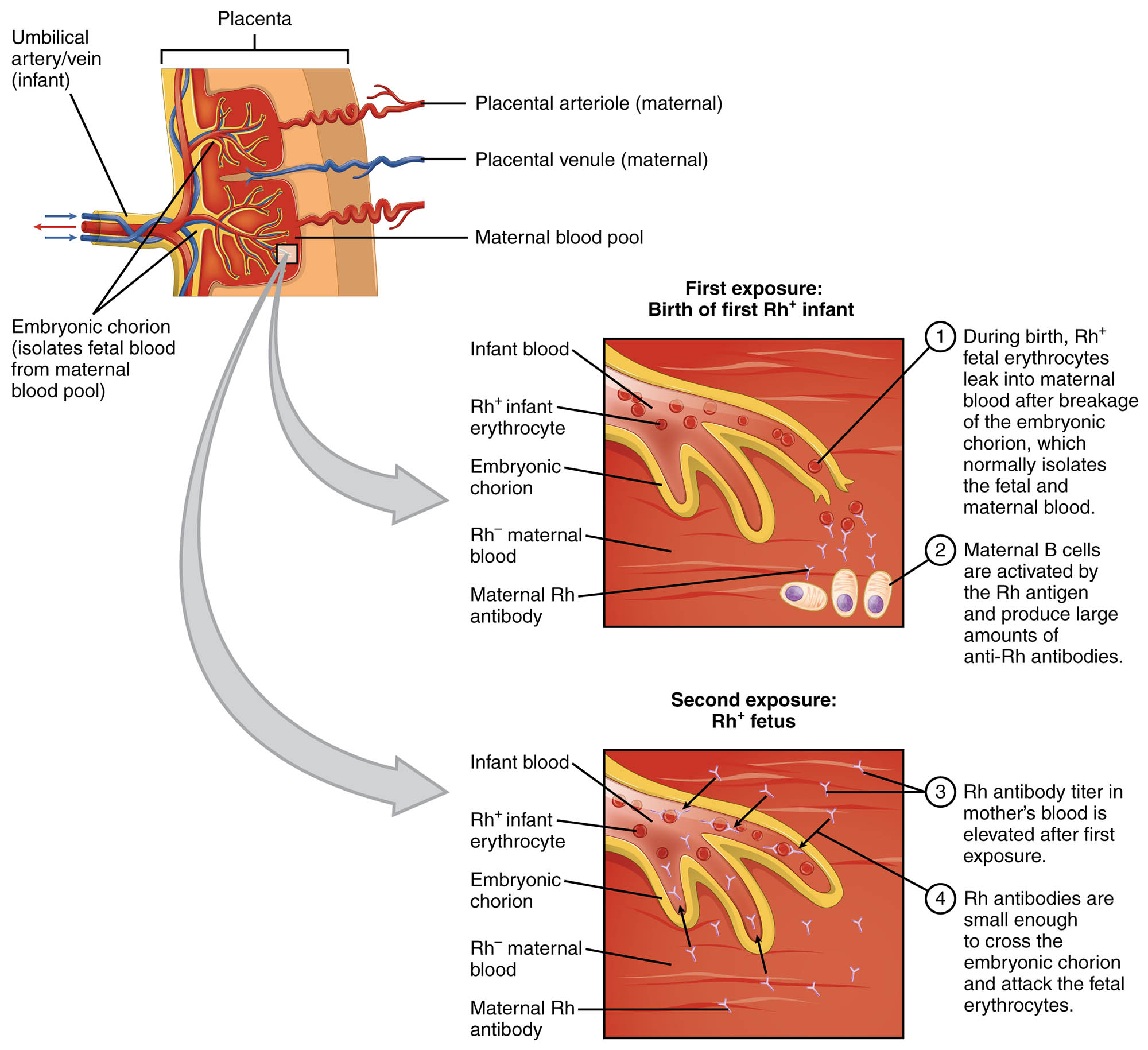
Erythroblastosis fetalis is a serious condition that arises when an Rh-negative mother carries an Rh-positive fetus, leading to potential complications in pregnancy. This article explores the underlying mechanisms depicted in the diagram, focusing on the sensitization process and the immune response that can harm the fetus. By examining the placental interaction and antibody activity, one can gain a deeper insight into this critical hematological disorder.
Umbilical vein (infant)
- The umbilical vein transports oxygenated blood from the placenta to the fetus, ensuring adequate oxygen supply during development.
- It plays a vital role in the fetal circulatory system, connecting the placenta to the fetal liver.
Umbilical artery (infant)
- The umbilical artery carries deoxygenated blood and waste products from the fetus back to the placenta for exchange.
- This vessel is essential for maintaining the fetal-placental circulation loop.
Placental arteriole (maternal)
- The placental arteriole delivers maternal blood to the placenta, facilitating the exchange of nutrients and oxygen.
- It branches into a network that interfaces with fetal blood in the placental structure.
Placental venule (maternal)
- The placental venule returns deoxygenated maternal blood from the placenta back to the maternal circulation.
- It ensures the continuous flow of maternal blood through the placental system.
Maternal blood pool
- The maternal blood pool within the placenta contains oxygenated blood that exchanges gases and nutrients with fetal blood.
- It is separated from fetal blood by the embryonic chorion, preventing direct mixing under normal conditions.
Embryonic chorion (isolates fetal from maternal blood pool)
- The embryonic chorion acts as a barrier, isolating fetal blood from maternal blood to prevent immune reactions.
- This structure can be breached during delivery or miscarriage, allowing fetal blood cells to enter the maternal circulation.
Rh⁺ infant erythrocyte
- Rh⁺ infant erythrocytes are red blood cells carrying the Rh antigen, which can trigger an immune response in an Rh⁻ mother.
- These cells may leak into maternal blood during the first pregnancy, initiating sensitization.
Embryonic chorion
- The embryonic chorion surrounds the fetus, contributing to the placental barrier that protects fetal blood.
- Its integrity is critical to preventing the mixing of maternal and fetal blood components.
Rh⁺ maternal blood
- Rh⁺ maternal blood indicates the presence of Rh antigens on the mother’s red blood cells, which is not typically a concern unless the fetus is Rh⁻.
- In the context of erythroblastosis fetalis, this label refers to the fetus’s Rh⁺ blood interacting with an Rh⁻ mother.
Maternal Rh antibody
- Maternal Rh antibodies are produced by an Rh⁻ mother after exposure to Rh⁺ fetal blood, targeting the Rh antigen.
- These antibodies can cross the placenta in subsequent pregnancies, leading to fetal red blood cell destruction.
Rh⁻ maternal blood
- Rh⁻ maternal blood lacks the Rh antigen, making the mother’s immune system reactive to Rh⁺ fetal cells.
- This blood type is central to the sensitization process in erythroblastosis fetalis.
Erythroblastosis fetalis, also known as hemolytic disease of the newborn, is a condition driven by an immunological mismatch between mother and fetus. The diagram illustrates how the first exposure to Rh⁺ erythrocytes during pregnancy or delivery sensitizes an Rh⁻ mother, prompting the production of anti-Rh antibodies. In a subsequent pregnancy with an Rh⁺ fetus, these antibodies can cross the placenta, causing agglutination and hemolysis of fetal red blood cells, which may result in severe anemia or even fetal loss.
First Exposure: Sensitization Process
The initial exposure marks the beginning of the sensitization process in an Rh⁻ mother. This stage sets the stage for potential complications in future pregnancies.
- During the birth of the first Rh⁺ infant, Rh⁺ erythrocytes may leak into the maternal blood, breaking the embryonic chorion barrier.
- The mother’s immune system recognizes the Rh antigen as foreign, activating B cells to produce large amounts of anti-Rh antibodies.
Second Exposure: Immune Response and Fetal Impact
The second exposure occurs when an Rh⁺ fetus is carried in a subsequent pregnancy, amplifying the immune reaction. This phase can lead to significant harm to the fetus.
- The elevated Rh antibody titer in the mother’s blood allows these antibodies to cross the embryonic chorion and attack fetal erythrocytes.
- The resulting hemolysis can cause severe anemia, jaundice, and, in severe cases, hydrops fetalis or fetal death.
Erythroblastosis fetalis is a condition rooted in the incompatibility between an Rh⁻ mother and an Rh⁺ fetus, primarily occurring due to blood group mismatches. The process begins with the placenta, a vital organ that facilitates the exchange of oxygen, nutrients, and waste between maternal and fetal blood. The umbilical vein and umbilical artery connect the fetus to the placenta, ensuring a continuous circulation of blood. The maternal blood pool, delivered via placental arterioles and returned through venules, remains separated from fetal blood by the embryonic chorion under normal circumstances. However, disruptions during delivery or miscarriage can allow fetal Rh⁺ erythrocytes to enter the maternal circulation.
The first exposure to Rh⁺ erythrocytes typically occurs during the birth of an Rh⁺ infant, where a small amount of fetal blood may leak into the Rh⁻ mother’s bloodstream. This breach activates the maternal immune system, leading to the production of anti-Rh antibodies by B cells. These antibodies do not immediately affect the first child but remain in the mother’s system, posing a risk in future pregnancies. The embryonic chorion, which normally isolates fetal and maternal blood, can be compromised, setting the stage for sensitization.
In a second pregnancy with an Rh⁺ fetus, the mother’s pre-existing anti-Rh antibodies pose a significant threat. The elevated antibody titer in the Rh⁻ maternal blood allows these immunoglobulins to cross the placenta, entering the fetal circulation. Once inside, the antibodies bind to Rh⁺ erythrocytes, causing agglutination and hemolysis, where red blood cells are destroyed. This process releases hemoglobin, which can lead to hyperbilirubinemia and kernicterus if untreated, affecting the fetus’s brain and other organs.
The severity of erythroblastosis fetalis depends on the extent of antibody crossing and the fetus’s ability to compensate for red blood cell loss. The condition can result in hemolytic anemia, characterized by increased erythropoiesis in the fetal liver and spleen, leading to the presence of immature erythrocytes called erythroblasts in the peripheral blood—hence the name. In severe cases, this can progress to hydrops fetalis, a life-threatening accumulation of fluid in the fetus, or intrauterine death. Early detection through maternal antibody screening and fetal monitoring is crucial.
Management of erythroblastosis fetalis often involves preventive measures and treatment. Administering Rh immunoglobulin (RhoGAM) to the Rh⁻ mother after the birth of an Rh⁺ child can prevent sensitization by neutralizing any fetal red blood cells before the immune response is triggered. In affected pregnancies, intrauterine transfusions of Rh⁻ blood to the fetus and phototherapy or exchange transfusions post-birth can mitigate severe outcomes. Understanding the anatomical and immunological dynamics depicted in the diagram is essential for grasping the clinical implications and therapeutic strategies.
Erythroblastosis fetalis underscores the delicate balance of the maternal-fetal interface. The placenta’s role in supporting fetal development while preventing immune conflicts is remarkable, yet vulnerable to disruption. By studying this condition, one gains valuable insights into the complexities of blood group compatibility and the body’s adaptive immune responses.

