This comprehensive guide explores the intricate pathways of cellular respiration, contrasting aerobic conditions with fermentation. We delve into how a single glucose molecule fuels the body, examining the energy-consuming and energy-releasing phases, and highlighting the critical differences in ATP production and end products.
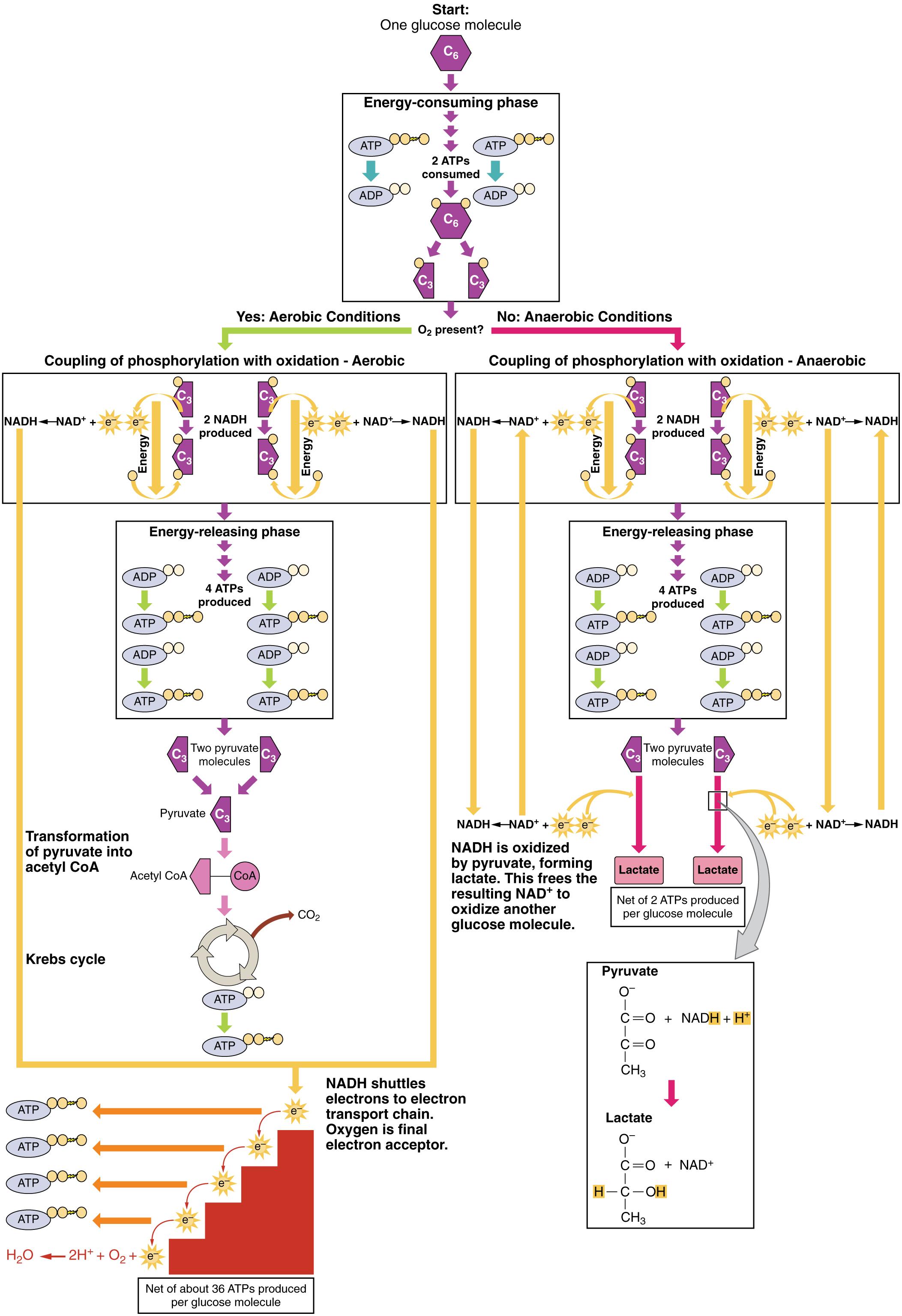
Start: One glucose molecule C6: This signifies the beginning of the entire process, where a six-carbon glucose molecule serves as the primary substrate for energy production. Glucose is a fundamental sugar that cells break down to generate usable energy in the form of ATP.
Energy-consuming phase: This initial phase of glycolysis requires an input of energy to prime the glucose molecule for subsequent breakdown. Two molecules of ATP are utilized to phosphorylate glucose, making it more reactive.
2 ATPS consumed: During the energy-consuming phase, two adenosine triphosphate molecules are hydrolyzed, donating phosphate groups to the glucose molecule. This investment of energy is crucial for destabilizing the glucose and facilitating its cleavage.
2 pyruvates produced: Following the energy-consuming and subsequent reactions, a single glucose molecule is ultimately broken down into two molecules of pyruvate. Each pyruvate molecule is a three-carbon compound, representing the product of glycolysis.
Yes: Aerobic Conditions: This pathway is activated when oxygen is present, indicating that the cell can proceed with the more efficient aerobic respiration. Oxygen acts as the final electron acceptor in the electron transport chain, enabling maximal ATP production.
No: Anaerobic Conditions: This pathway is activated in the absence of oxygen, indicating that the cell must rely on fermentation to regenerate NAD+ for glycolysis to continue. While less efficient, anaerobic conditions allow for rapid energy production in situations of oxygen scarcity.
Coupling of phosphorylation with oxidation – Aerobic: This represents the intricate link between the phosphorylation of ADP to ATP and the oxidation of molecules like NADH during aerobic respiration. This coupling is fundamental to how cells efficiently capture energy released from nutrient breakdown.
Coupling of phosphorylation with oxidation – Anaerobic: In anaerobic conditions, a similar coupling occurs, but the electron acceptor is an organic molecule rather than oxygen. This allows for the regeneration of NAD+ from NADH, which is essential for sustained glycolysis in the absence of oxygen.
2 NADH produced: During glycolysis, in both aerobic and anaerobic conditions, two molecules of nicotinamide adenine dinucleotide (NADH) are generated. NADH is a crucial electron carrier that plays a vital role in transferring electrons to other parts of the respiratory pathway.
Energy-releasing phase: This subsequent phase of glycolysis generates ATP, recouping the initial energy investment and yielding a net gain. Through a series of enzymatic reactions, energy is captured from the breaking bonds within the glucose derivatives.
4 ATPS produced: In the energy-releasing phase of glycolysis, four molecules of ATP are synthesized via substrate-level phosphorylation. This directly contributes to the cell’s immediate energy supply.
Transformation of pyruvate into acetyl CoA: Under aerobic conditions, the pyruvate molecules produced from glycolysis are transported into the mitochondria and converted into acetyl-CoA. This conversion is a crucial preparatory step before the Krebs cycle, linking glycolysis to the subsequent stages of aerobic respiration.
Krebs cycle: Also known as the citric acid cycle, the Krebs cycle is a central metabolic pathway in aerobic organisms that oxidizes acetyl-CoA, generating electron carriers like NADH and FADH2, and a small amount of ATP. This cycle occurs in the mitochondrial matrix and is a major hub for energy production.
NADH shuttles electrons to electron transport chain. Oxygen is final electron acceptor: This describes the critical role of NADH in delivering high-energy electrons to the electron transport chain, where they are progressively passed down a series of protein complexes. Ultimately, oxygen accepts these electrons, forming water, a process that drives the synthesis of a large amount of ATP.
Net of about 36 ATPS produced per glucose molecule: This figure represents the substantial energy yield from aerobic respiration, encompassing glycolysis, the Krebs cycle, and oxidative phosphorylation. This high ATP production is characteristic of efficient energy extraction when oxygen is abundant.
NADH is oxidized by pyruvate, forming lactate. This frees the resulting NAD+ to oxidize another glucose molecule: In anaerobic conditions, pyruvate accepts electrons from NADH, regenerating NAD+ and forming lactate. This critical step allows glycolysis to continue, providing a continuous supply of ATP even without oxygen.
Net of 2 ATPS produced per glucose molecule: This signifies the much lower energy yield of fermentation compared to aerobic respiration. While sufficient for short bursts of energy or in oxygen-deprived environments, it highlights the inefficiency of anaerobic metabolism.
Lactate: Lactate is the end product of lactic acid fermentation in humans and some other organisms. Its accumulation can lead to muscle fatigue and soreness during intense exercise when oxygen supply is insufficient.
Pyruvate: Pyruvate is a key intermediate molecule in metabolism, serving as the branch point between aerobic respiration and fermentation. Its fate depends on the availability of oxygen within the cell.
Lactate: In the context of fermentation, lactate is the final product formed when pyruvate is reduced by NADH. This process regenerates NAD+, which is essential for sustaining glycolysis and ATP production under anaerobic conditions.
Cellular respiration is a fundamental biological process that converts nutrients into adenosine triphosphate (ATP), the primary energy currency of the cell. This intricate mechanism ensures that all living organisms, from single-celled bacteria to complex multicellular beings, have the energy required to sustain life processes. The diagram above illustrates two major pathways for glucose metabolism: aerobic respiration, which occurs in the presence of oxygen, and fermentation, which proceeds in the absence of oxygen. Understanding these pathways is crucial for comprehending how our bodies generate energy for everything from muscle contraction to brain function.
Our bodies are incredibly adaptable, capable of producing energy through different routes depending on oxygen availability. This diagram succinctly outlines these two distinct yet interconnected processes, starting with a single glucose molecule. It highlights the initial energy-consuming phase, where two ATPs are invested, leading to the production of two pyruvate molecules. From this crucial juncture, the fate of pyruvate diverges based on the presence or absence of oxygen, leading to vastly different energy yields and end products. The efficiency of energy extraction is a key differentiator between these pathways, with profound implications for cellular function.
In aerobic conditions, the presence of oxygen allows for a highly efficient energy extraction process. The pyruvate molecules undergo further transformation, leading into the Krebs cycle and the electron transport chain. This pathway maximizes ATP production, generating approximately 36 ATP molecules per glucose molecule. Conversely, in anaerobic conditions, or within cells like erythrocytes that lack mitochondria, fermentation steps in. This process, while far less efficient, generating only 2 ATPs per glucose, is vital for rapid energy production when oxygen is scarce. For instance, during intense physical activity, muscle cells may temporarily switch to fermentation to meet immediate energy demands.
- Aerobic respiration yields significantly more ATP.
- Fermentation regenerates NAD+ for glycolysis to continue.
- Pyruvate is a central intermediate in both pathways.
- Oxygen is the final electron acceptor in aerobic respiration.
The human body is a marvel of biochemical engineering, constantly adapting its metabolic strategies to meet fluctuating energy demands. The choice between aerobic respiration and fermentation is dictated primarily by the availability of oxygen. When oxygen is abundant, as during rest or moderate exercise, the body efficiently breaks down glucose through aerobic respiration. This process, occurring mainly in the mitochondria, involves the Krebs cycle and oxidative phosphorylation, ultimately producing a substantial amount of ATP, carbon dioxide, and water. This highly efficient pathway is the body’s preferred method for sustained energy production.
However, there are times when oxygen supply cannot keep pace with demand, such as during strenuous, high-intensity exercise. In these situations, muscle cells switch to anaerobic respiration, specifically lactic acid fermentation. This pathway allows glycolysis to continue, producing a small but rapid supply of ATP, which is crucial for short bursts of intense activity. During this process, pyruvate is converted into lactate, regenerating NAD+ from NADH. While lactate accumulation can lead to muscle fatigue and soreness, it is a vital mechanism for temporary energy generation in oxygen-deprived environments.
Understanding the nuances of aerobic respiration and fermentation is fundamental to comprehending human physiology and various medical conditions. For example, in conditions like anemia, where oxygen delivery to tissues is impaired, cells may increasingly rely on anaerobic metabolism, leading to a buildup of lactate. Similarly, in certain metabolic disorders, the efficiency of these pathways can be compromised, impacting overall energy production and cellular health. The balance and interplay between these two pathways are critical for maintaining cellular homeostasis and overall organismal well-being.
In conclusion, the breakdown of a single glucose molecule represents a complex and highly regulated process with two main branches: aerobic respiration and fermentation. Each pathway is uniquely adapted to specific cellular conditions and oxygen availability, demonstrating the remarkable flexibility of biological systems. While aerobic respiration provides a highly efficient and sustainable source of ATP, fermentation offers a rapid, albeit less efficient, alternative when oxygen is limited. Both pathways are indispensable for life, underscoring the intricate balance and adaptability of cellular metabolism in maintaining energy homeostasis within the human body.

