Discover the fundamental principles of Boyle’s Law and its critical role in human respiration. This article delves into how changes in gas volume directly impact pressure, explaining the mechanics behind every breath you take.
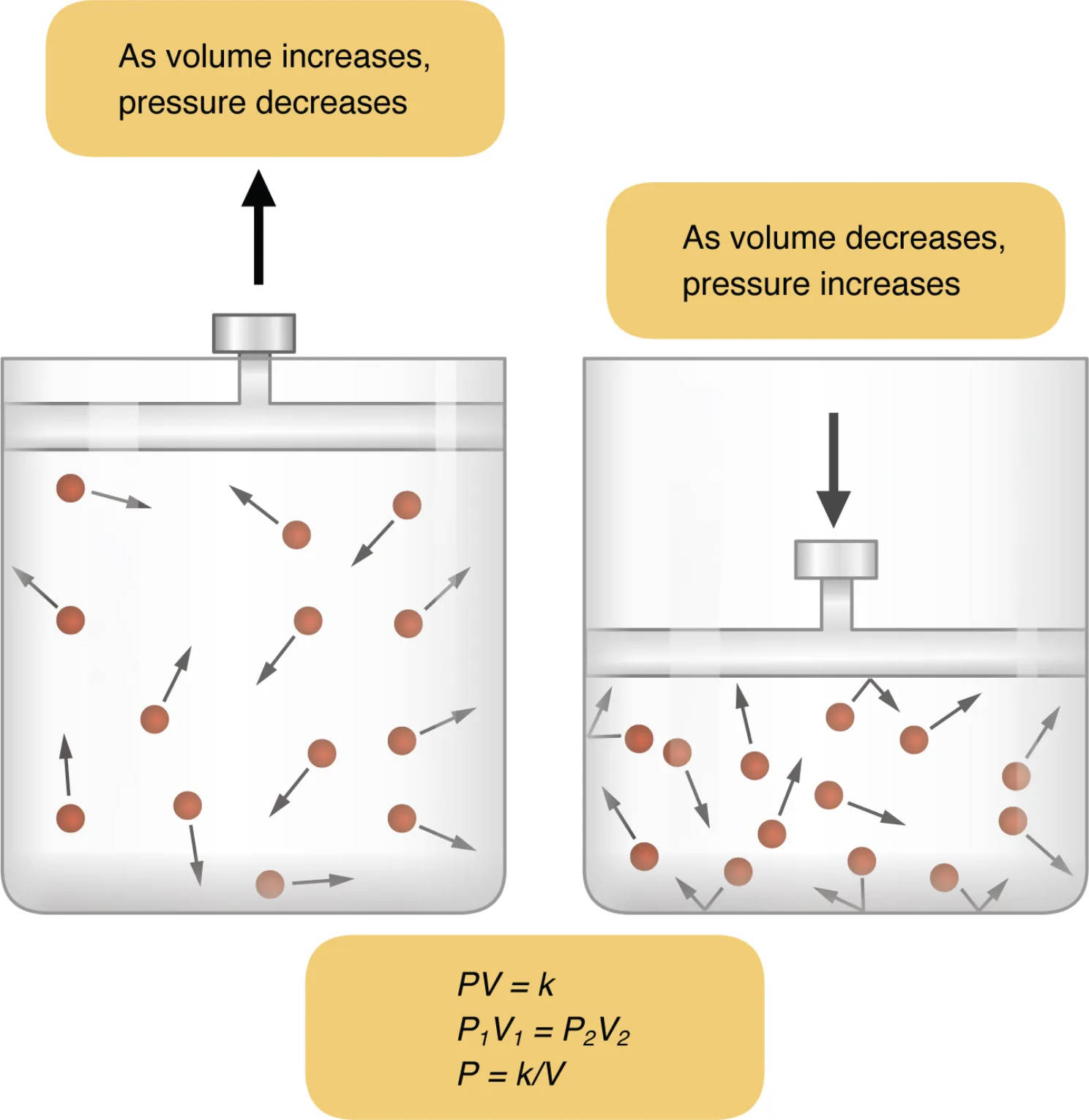
As volume increases, pressure decreases: This label indicates a state where the piston is moving upwards, expanding the container’s volume. As the volume of the gas increases, the gas particles have more space to move around, leading to fewer collisions with the container walls and thus a reduction in pressure. This principle is vital in understanding how our lungs expand to draw air in during inhalation.
As volume decreases, pressure increases: Conversely, this label illustrates the piston moving downwards, compressing the gas into a smaller volume. When the volume decreases, the gas particles are forced into a tighter space, resulting in more frequent collisions with the container walls and a consequent increase in pressure. This mechanism is analogous to how our lungs contract to expel air during exhalation.
PV = k, P1V1 = P2V2, P = k/V: These equations represent the mathematical formulation of Boyle’s Law. PV = k signifies that for a fixed amount of gas at constant temperature, the product of its pressure (P) and volume (V) remains constant (k). P1V1 = P2V2 extends this, showing that the initial pressure and volume product equals the final pressure and volume product, while P = k/V explicitly states the inverse relationship between pressure and volume. These formulas are fundamental to quantitative analysis in gas dynamics and respiratory physiology.
The intricate dance between pressure and volume is a cornerstone of physics, particularly in the study of gases. One of the most foundational principles governing this relationship is Boyle’s Law, named after the 17th-century Anglo-Irish chemist Robert Boyle. This law elucidates the inverse proportionality between the absolute pressure and volume of a given mass of an ideal gas when the temperature and the number of gas particles are kept constant. In simpler terms, if you increase the volume of a container holding a gas, its pressure will decrease, and vice versa. This principle is not merely an abstract concept confined to textbooks; it has profound implications, especially in the realm of human physiology.
Understanding Boyle’s Law is crucial for comprehending various natural phenomena and technological applications. From the functioning of internal combustion engines to the behavior of weather systems, its influence is widespread. However, perhaps one of the most remarkable and immediate applications of Boyle’s Law can be observed within our own bodies: the process of respiration. Every breath we take, every inhalation and exhalation, is a direct consequence of this fundamental gas law at work. Without the precise interplay of pressure and volume changes dictated by Boyle’s Law, our ability to draw oxygen into our lungs and expel carbon dioxide would be impossible.
The elegance of Boyle’s Law lies in its simplicity and its powerful explanatory capacity. It allows us to predict how gases will behave under varying conditions, providing a critical framework for scientific inquiry. In the context of human biology, this understanding bridges the gap between the microscopic world of gas molecules and the macroscopic function of our respiratory system.
- Pressure-Volume Relationship: Explains how changes in the space a gas occupies affect the force it exerts.
- Constant Temperature: Emphasizes that this relationship holds true under isothermal conditions.
- Respiratory Mechanics: Directly applies to the process of breathing, illustrating lung expansion and contraction.
- Gas Exchange: Indirectly supports the understanding of how gases move into and out of the bloodstream.
The human respiratory system is a marvel of biological engineering, meticulously designed to facilitate the continuous exchange of gases vital for life. At its core, the mechanics of breathing are governed by Boyle’s Law. During inhalation, the diaphragm contracts and moves downwards, while the intercostal muscles contract, pulling the rib cage upwards and outwards. This coordinated muscular action significantly increases the volume of the thoracic cavity, and consequently, the volume of the lungs. As the lung volume expands, the pressure within the alveoli—the tiny air sacs in the lungs—decreases. According to Boyle’s Law, this reduction in intrapulmonary pressure creates a pressure gradient, causing atmospheric air, which is at a higher pressure, to flow into the lungs until the pressure inside and outside equalizes. This influx of air brings in the much-needed oxygen that our bodies require for metabolic processes.
Conversely, exhalation is largely a passive process during quiet breathing, though it can be active during strenuous activities. The diaphragm relaxes and moves upwards, and the intercostal muscles relax, allowing the rib cage to move downwards and inwards. This action reduces the volume of the thoracic cavity and the lungs. As lung volume decreases, the pressure within the alveoli increases, again due to Boyle’s Law. This elevated intrapulmonary pressure now exceeds the atmospheric pressure, creating a gradient that forces air, rich in carbon dioxide, out of the lungs. This continuous cycle of inhalation and exhalation ensures that our bodies are constantly supplied with oxygen and rid of metabolic waste products. The efficiency of this process is paramount for maintaining homeostasis and supporting all physiological functions, from cellular respiration to cognitive activities.
In conclusion, Boyle’s Law provides an indispensable framework for understanding the fundamental mechanics of human respiration. The inverse relationship between gas pressure and volume is not just a theoretical concept but a tangible principle that underpins every breath we take. By comprehending how changes in thoracic volume lead to corresponding alterations in intrapulmonary pressure, we gain a deeper appreciation for the intricate design and flawless execution of our respiratory system. This understanding is critical not only for medical professionals and scientists but also for anyone interested in the remarkable workings of the human body.

