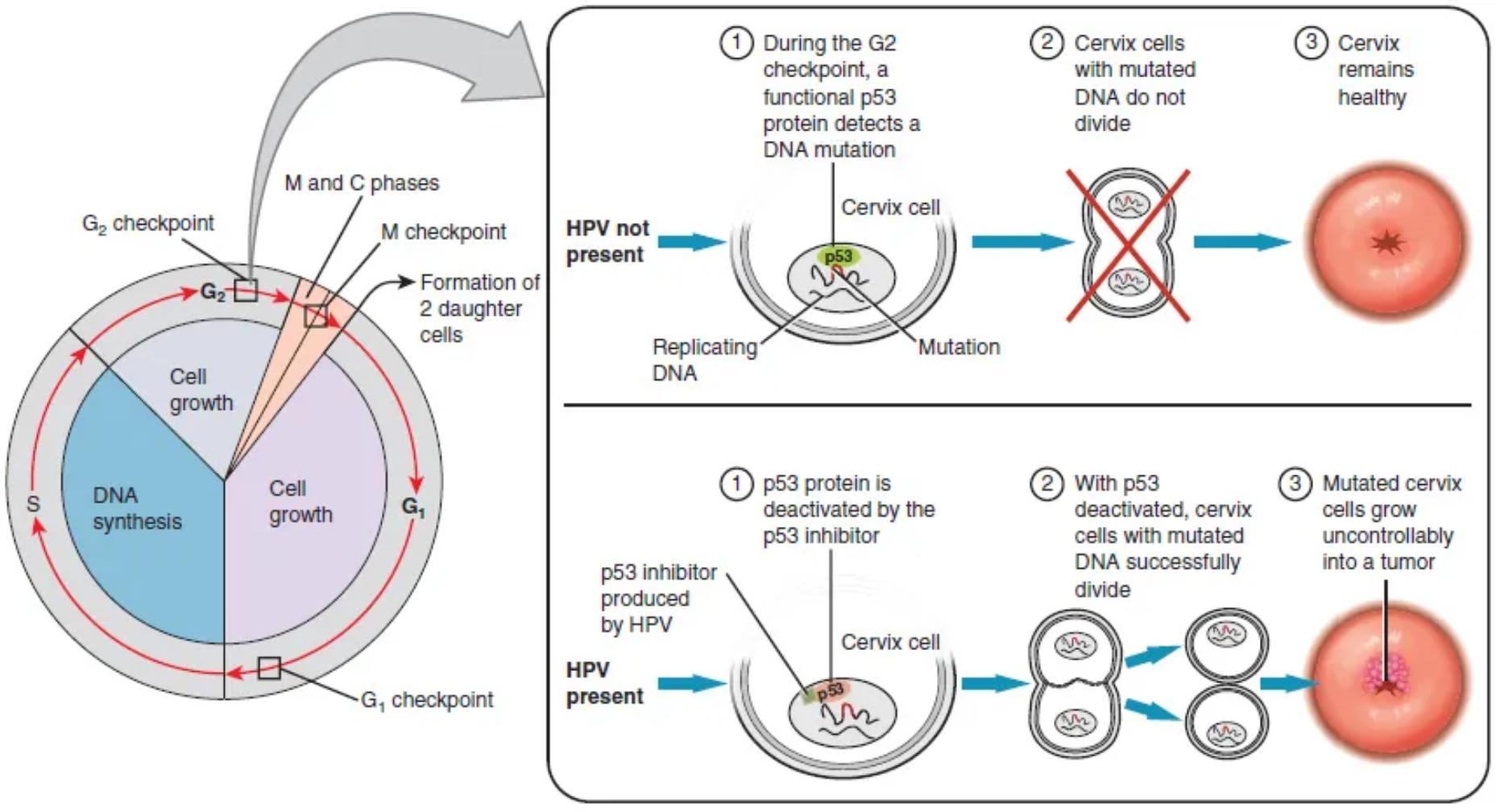
Cervical cancer, a significant global health concern, is primarily caused by persistent infection with high-risk human papillomavirus (HPV). This comprehensive diagram illustrates how HPV can disrupt crucial cellular checkpoints, particularly by inactivating the tumor suppressor protein p53, leading to uncontrolled cell growth and tumor formation. Understanding this molecular mechanism is vital for appreciating cancer prevention strategies, including vaccination and screening.
G2 checkpoint: The G2 checkpoint is a critical control point in the eukaryotic cell cycle that occurs at the end of the G2 phase, before entry into mitosis. Its function is to ensure that DNA replication is complete and that the DNA is not damaged, preventing flawed cells from dividing.
M and C phases: The M phase (Mitosis) is the stage of the cell cycle where the nucleus divides, followed by the C phase (Cytokinesis), where the cytoplasm divides, resulting in two daughter cells. These phases represent the actual cell division process.
M checkpoint: The M checkpoint, also known as the spindle assembly checkpoint, occurs during metaphase of mitosis. It ensures that all chromosomes are correctly attached to the spindle microtubules before sister chromatids separate, preventing aneuploidy.
Formation of 2 daughter cells: This represents the successful completion of cell division (mitosis and cytokinesis), where one parent cell gives rise to two genetically identical daughter cells. This process is essential for growth and tissue repair.
Cell growth: Cell growth refers to the increase in the size of a cell, primarily occurring during the G1 and G2 phases of the cell cycle. During these periods, the cell synthesizes proteins and organelles in preparation for division.
DNA synthesis: DNA synthesis, or replication, occurs during the S phase of the cell cycle. During this phase, the cell duplicates its entire genome, ensuring that each daughter cell receives a complete set of chromosomes.
G1 checkpoint: The G1 checkpoint is a major control point in the cell cycle that monitors cell size, nutrient availability, growth factors, and DNA integrity. It determines whether the cell should proceed to DNA replication (S phase) or enter a quiescent state (G0).
During the G2 checkpoint, a functional p53 protein detects a DNA mutation: In a healthy cervical cell, if DNA damage or mutation occurs, the p53 protein becomes activated at the G2 checkpoint. This activation allows p53 to halt the cell cycle and initiate DNA repair mechanisms.
Cervix cell: This represents an epithelial cell from the cervix, which is the target cell type for HPV infection and the origin of cervical cancer. These cells normally undergo controlled division and differentiation.
Replicating DNA: This indicates the DNA within the cervical cell nucleus undergoing the process of duplication during the S phase of the cell cycle. Errors during this replication can lead to mutations.
Mutation: A mutation refers to a change in the DNA sequence of a cell. Such changes can alter gene function, potentially leading to uncontrolled cell growth if tumor suppressor genes are affected or oncogenes are activated.
Cervix cells with mutated DNA do not divide: When a functional p53 protein detects mutated DNA, it prevents the cell from progressing through the cell cycle and dividing. This crucial mechanism protects against the propagation of damaged cells.
Cervix remains healthy: The ability of p53 to halt the division of cells with mutated DNA ensures that such damaged cells are either repaired or undergo apoptosis (programmed cell death). This maintains the integrity and health of the cervical tissue, preventing cancer.
p53 protein is deactivated by the p53 inhibitor produced by HPV: High-risk HPV strains produce viral oncoproteins, such as E6, which act as a p53 inhibitor. This viral protein directly binds to and deactivates or degrades the p53 tumor suppressor protein, thereby removing a critical cellular safeguard.
With p53 deactivated, cervix cells with mutated DNA successfully divide: When p53 is deactivated by HPV, cervical cells containing mutated DNA lose their crucial checkpoint control. They are no longer prevented from dividing and can bypass the G2 checkpoint, allowing the damaged genetic material to be passed on.
Mutated cervix cells grow uncontrollably into a tumor: The continuous division of cervical cells with mutated DNA, unchecked by the deactivated p53, leads to an accumulation of genetic errors and uncontrolled proliferation. This unchecked growth ultimately results in the formation of a malignant tumor, which is cervical cancer.
Cervical Cancer: The Molecular Link to HPV
Cervical cancer is a malignancy that originates in the cells of the cervix, the lower part of the uterus that connects to the vagina. Globally, it remains a significant public health challenge, primarily attributable to persistent infection with high-risk strains of the human papillomavirus (HPV). While most HPV infections are cleared naturally by the immune system, some persist and can lead to precancerous changes and, eventually, invasive cancer. The diagram visually explains the cellular and molecular mechanisms underlying this progression, focusing on how HPV subverts a critical tumor suppressor pathway.
The development of cancer is often a multi-step process involving the accumulation of genetic mutations and the breakdown of normal cellular control mechanisms. In the context of cervical cancer, HPV plays a pivotal role by interfering with the cell cycle, particularly by targeting key proteins that regulate cell growth and division. Understanding this interaction provides insight into why some HPV infections progress to cancer, while others do not.
Key elements in this pathological process include:
- The normal functioning of cell cycle checkpoints, such as the G2 checkpoint.
- The vital role of the p53 tumor suppressor protein in detecting and responding to DNA damage.
- The mechanism by which HPV’s oncoproteins (e.g., E6) inactivate p53.
- The consequence of p53 deactivation: uncontrolled cell proliferation and tumor formation.
This molecular understanding forms the basis for current cervical cancer prevention strategies, including HPV vaccination and regular screening tests like the Pap test.
Cell Cycle Control and HPV’s Oncogenic Strategy
The cell cycle is a tightly regulated process that ensures accurate DNA replication and cell division. Critical checkpoints, such as the G1, S, and G2 checkpoints, act as internal monitors, halting progression if DNA damage or other abnormalities are detected. A crucial player in these checkpoints, particularly at G2, is the tumor suppressor protein p53. In healthy cervical cells, if a DNA mutation occurs during replication or due to external factors, a functional p53 protein identifies this damage. Upon detection, p53 can trigger several responses: it can halt the cell cycle to allow for DNA repair, or if the damage is too extensive, it can initiate apoptosis (programmed cell death) to eliminate the damaged cell. This mechanism is essential for maintaining genomic stability and preventing the proliferation of potentially cancerous cells, thus keeping the cervix healthy.
However, high-risk strains of HPV have evolved a cunning strategy to bypass this cellular defense. These viruses produce viral oncoproteins, notably E6 and E7. The diagram specifically highlights the role of an HPV-produced p53 inhibitor, which is the E6 oncoprotein. E6 directly binds to and deactivates or promotes the degradation of the p53 protein. With p53 effectively neutralized, cervical cells with mutated or damaged DNA lose their crucial checkpoint control. They can now successfully bypass the G2 checkpoint and other regulatory points, continuing to divide despite genetic abnormalities. This unchecked proliferation of genetically compromised cervical cells leads to an accumulation of further mutations and ultimately results in their uncontrolled growth, forming a malignant tumor—cervical cancer. This disruption of normal cell cycle regulation by HPV is the primary molecular driver of cervical carcinogenesis.
In conclusion, the development of cervical cancer is a direct consequence of high-risk HPV’s ability to interfere with fundamental cell cycle checkpoints and inactivate critical tumor suppressor proteins like p53. By dismantling these cellular safeguards, HPV allows damaged and mutated cervical cells to proliferate uncontrollably, leading to tumor formation. This profound understanding of the molecular pathogenesis of cervical cancer underscores the immense value of HPV vaccination as a primary prevention strategy and the ongoing importance of regular cervical cancer screening programs.

