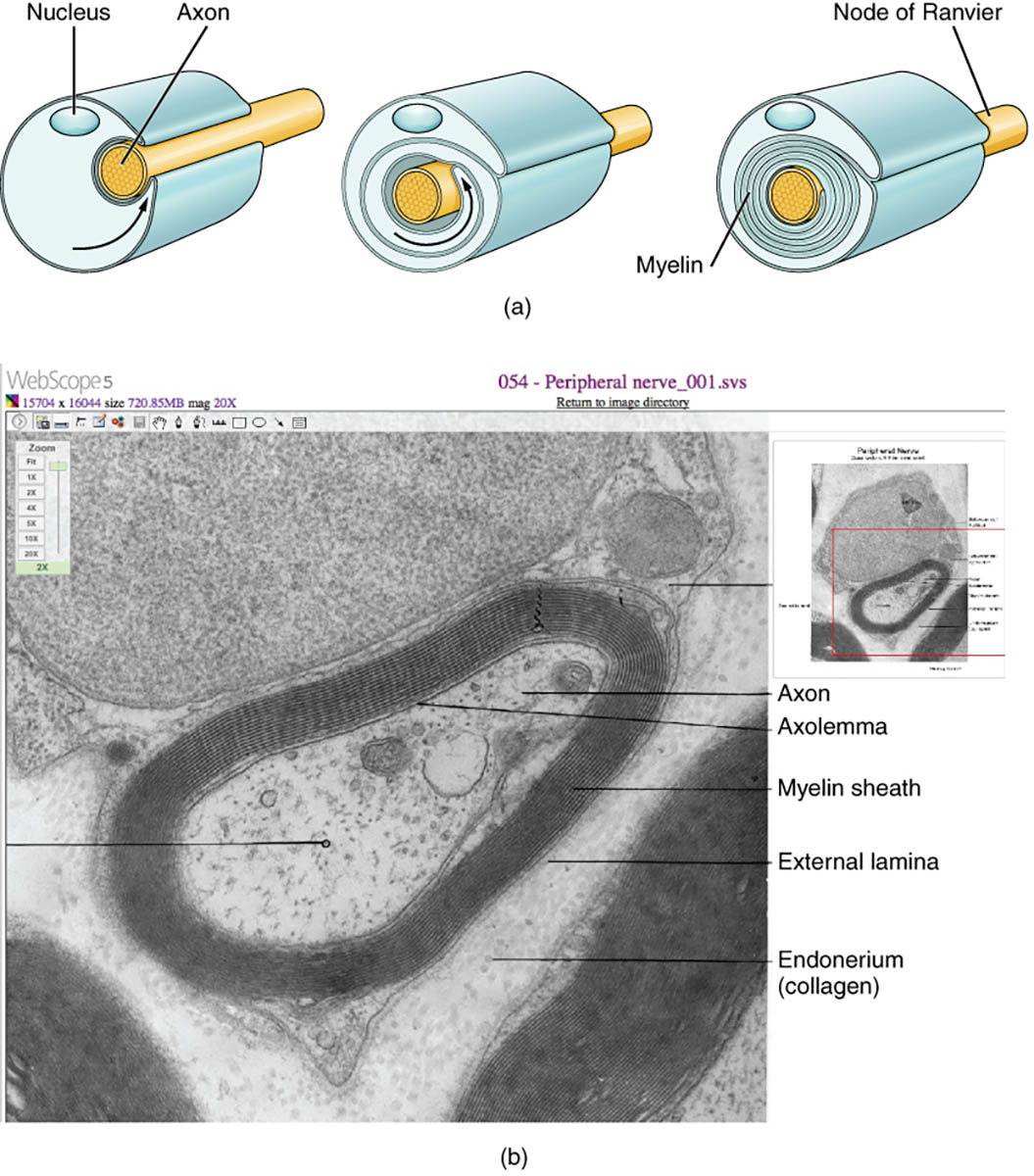
Myelination represents a critical adaptation in the nervous system, where glial cells wrap layers of membrane around axons to enhance signal transmission speed and efficiency, fundamentally supporting rapid neural communication. This image illustrates the myelination process in both schematic and microscopic views, showcasing how Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS) form insulating sheaths, with detailed labels highlighting key structures like the myelin sheath and node of Ranvier. Such insulation enables saltatory conduction, where action potentials jump between nodes, accelerating impulses up to 100 times faster than in unmyelinated fibers, essential for coordinated movements, sensory processing, and cognitive functions.
Labeled Structures in Myelination
Nucleus
The nucleus of the glial cell is shown in the schematic as a central structure within the Schwann cell, containing genetic material that directs myelin protein synthesis. It regulates the production of lipids and proteins like myelin basic protein (MBP) necessary for compact sheath formation.
Axon
The axon appears as the central yellow core in the diagram and a dark tubular structure in the micrograph, serving as the conduit for electrical impulses from the neuron soma to synapses. It is enveloped by myelin to prevent signal leakage and support metabolic exchanges through axolemma channels.
Node of Ranvier
The node of Ranvier is depicted as gaps between myelin segments, where the axon is exposed and enriched with voltage-gated sodium channels. These nodes facilitate the regeneration of action potentials during saltatory conduction, ensuring energy-efficient and rapid nerve impulse propagation.
Myelin
Myelin is illustrated in cross-section as concentric layers spiraling around the axon, composed of lipid-rich glial membrane extensions. This fatty insulation reduces capacitance and increases resistance, allowing faster electrical signaling while protecting the axon from environmental stressors.
Axolemma
The axolemma labels the axon’s plasma membrane in the micrograph, directly interfacing with the innermost myelin layer. It contains ion pumps and transporters, such as Na+/K+-ATPase, crucial for maintaining resting potential and enabling depolarization at nodes.
Myelin sheath
The myelin sheath encompasses the multilayered wrapping visible in both views, formed by repeated glial membrane coils that compact into major dense and intraperiod lines. In the PNS, a single Schwann cell forms one sheath segment, providing structural stability and trophic support to the underlying axon.
External lamina
The external lamina appears as a thin basal layer surrounding the myelin sheath in the electron micrograph, consisting of extracellular matrix components like laminin and collagen IV. It anchors the Schwann cell to surrounding tissues and facilitates nutrient diffusion while aiding in nerve regeneration post-injury.
Endoneurium (collagen)
The endoneurium, labeled with collagen, refers to the loose connective tissue matrix embedding individual nerve fibers, rich in type I and III collagen fibers. This layer provides mechanical support, houses capillaries for nourishment, and creates a permissive environment for axonal growth during repair.
In-Depth Anatomy of Myelination
The anatomy of myelinated axons reveals specialized adaptations for optimal neural performance. Structural details emphasize the collaborative role of glia and neurons.
- In the PNS, Schwann cells initiate myelination by extending a mesaxon that spirals around the axon, compacting cytoplasm out to form mature myelin.
- CNS oligodendrocytes differ by extending multiple processes, each myelinating separate axon segments, up to 40 per cell, allowing dense packing in white matter tracts.
- The myelin sheath’s g-ratio, typically 0.6-0.8, balances axon diameter with insulation thickness for maximal conduction velocity.
- Nodes of Ranvier span about 1-2 micrometers, flanked by paranodal loops where myelin attaches via septate-like junctions involving caspr and contactin proteins.
- The axolemma at internodes is sparsely channeled, conserving energy by limiting ion fluxes to nodal regions.
Physiological Mechanisms of Myelination
Myelination profoundly influences axonal physiology through electrochemical enhancements. Processes involve dynamic glial-axonal signaling for development and maintenance.
- Saltatory conduction jumps action potentials from node to node, propelled by high nodal sodium channel density (Nav1.6 isoforms), reducing propagation time.
- Myelin lipids, primarily galactocerebroside and cholesterol, create a hydrophobic barrier that minimizes current loss across the internode.
- Oligodendrocytes and Schwann cells provide metabolic support via monocarboxylate transporters (MCT1), shuttling lactate to axons for ATP production during high activity.
- Developmental myelination is activity-dependent, with neuronal firing releasing ATP that stimulates glial wrapping via purinergic receptors.
- In adults, myelin plasticity allows adaptive changes, such as thickening in response to learning-induced circuit strengthening.
Differences Between PNS and CNS Myelination
Myelination varies between peripheral and central systems, reflecting distinct glial types and regenerative capacities. These differences impact function and pathology responses.
- PNS Schwann cells myelinate one axon segment each, enabling robust regeneration as they dedifferentiate post-injury to form Bands of Büngner for guidance.
- CNS oligodendrocytes myelinate multiple segments but have limited remyelination potential due to inhibitory factors like Nogo-A from debris.
- Peripheral myelin includes Schmidt-Lanterman incisures, cytoplasmic channels for nutrient flow, absent in CNS myelin for tighter compaction.
- External lamina in PNS provides a basal membrane scaffold, contrasting with CNS perinodal astrocyte processes that form glial limitans.
- Collagen-rich endoneurium in PNS cushions fibers within fascicles, while CNS lacks equivalent connective tissue, relying on pia mater boundaries.
Developmental and Molecular Aspects
Myelination unfolds through orchestrated genetic and environmental cues during growth. Molecular pathways govern glial differentiation and wrapping.
- In embryos, neural crest-derived Schwann cells associate with axons under neuregulin-1 signaling from ErbB receptors, transitioning from promyelinating to myelinating states.
- CNS oligodendrocyte progenitors (OPCs) migrate from ventricular zones, proliferating via PDGF-AA before maturing under thyroid hormones like T3 and T4 that promote MBP expression.
- Transcription factors such as Olig2 and Sox10 coordinate myelin gene activation, ensuring timely sheath formation post-birth in humans.
- Axonal caliber thresholds (about 1 μm) trigger myelination, with larger diameters correlating to thicker sheaths for faster conduction in motor nerves.
- Epigenetic modifications, like histone acetylation, regulate myelin repair in adulthood, influenced by aging or disease.
Research and Imaging Techniques
Advanced imaging and models deepen insights into myelination dynamics. Techniques capture ultrastructure and function in vivo.
- Electron microscopy, as in the provided micrograph (EM × 1,460,000), reveals nanoscale myelin lamellae and axolemma interfaces, quantifying layer counts.
- Diffusion tensor imaging (DTI) maps white matter tracts non-invasively, assessing myelin integrity via fractional anisotropy metrics.
- Live-cell imaging with fluorescent reporters tracks OPC process extension and wrapping in zebrafish models.
- Proteomics identifies myelin components like PLP1, linking mutations to disorders via mass spectrometry.
- Computational simulations model saltatory conduction using Hodgkin-Huxley equations adapted for nodal parameters.
Clinical Relevance and Related Pathologies
While the image depicts normal myelination, disruptions lead to significant disorders. Understanding these informs diagnostic and therapeutic strategies.
- Demyelination in multiple sclerosis (MS) targets CNS oligodendrocytes, causing plaque formation and slowed conduction, managed with immunomodulators like ocrelizumab.
- Peripheral neuropathies like Charcot-Marie-Tooth involve Schwann cell defects, with PMP22 duplications leading to hypertrophic myelin and weakness.
- Guillain-Barré syndrome acutely demyelates PNS axons via autoimmune attacks, presenting with ascending paralysis treatable by plasmapheresis.
- Congenital hypomyelination, as in Pelizaeus-Merzbacher disease, stems from PLP1 mutations, impairing oligodendrocyte function and causing spastic paraplegia.
- Traumatic injuries benefit from PNS remyelination, enhanced by therapies like electrical stimulation to promote Schwann proliferation.
In conclusion, the myelination process, as vividly captured in this schematic and electron micrograph, underscores the elegant partnership between axons and glial cells, optimizing nervous system efficiency across PNS and CNS. Appreciating these mechanisms not only illuminates fundamental neuroscience but also paves the way for interventions in demyelinating conditions, advancing toward restored neural function and improved patient outcomes.

