Explore the intricate balance between catabolic and anabolic pathways, essential for converting nutrients into usable energy and building complex molecules. This article details how glucose, amino acids, and fats are metabolized, highlighting their roles in glycolysis, the Krebs cycle, and the electron transport chain.
Catabolic: Catabolic pathways involve the breakdown of complex molecules into simpler ones, typically releasing energy in the process. These reactions are crucial for energy production, as they convert dietary nutrients and stored molecules into ATP.
Anabolic: Anabolic pathways involve the synthesis of complex molecules from simpler precursors, requiring an input of energy. These reactions are essential for growth, repair, and the storage of energy, such as the formation of glycogen or triglycerides.
Glycogen: Glycogen is a branched polysaccharide of glucose, serving as the primary form of glucose storage in animals, mainly in the liver and muscles. It is readily mobilized to provide glucose when energy is needed.
Glycogenolysis: Glycogenolysis is the biochemical pathway that breaks down glycogen into glucose, primarily occurring in the liver and muscle cells. This process is crucial for maintaining blood glucose levels and providing energy for muscle contraction.
Glycogenesis: Glycogenesis is the metabolic pathway that synthesizes glycogen from glucose, primarily occurring in the liver and muscles when glucose is abundant. This process allows the body to store excess glucose for future energy needs.
Glucose C6: Glucose is a simple six-carbon sugar, serving as the body’s primary and preferred source of energy. It is metabolized through glycolysis to produce ATP, fueling various cellular activities.
Glycerol: Glycerol is a three-carbon alcohol that forms the backbone of triglycerides, and it is released during the breakdown of fats. It is a glucogenic molecule, meaning it can be converted into glucose or enter glycolysis to produce energy.
Glycolysis: Glycolysis is the metabolic pathway that converts glucose into pyruvate, occurring in the cytoplasm of virtually all cells. This process generates a net of two ATP molecules and two NADH molecules, crucial for subsequent energy production.
Gluconeogenesis: Gluconeogenesis is the metabolic pathway that synthesizes glucose from non-carbohydrate precursors, such as lactate, certain amino acids, and glycerol. This process is vital for maintaining blood glucose homeostasis, especially during fasting.
Pyruvate molecules C3: Pyruvate is a three-carbon alpha-keto acid that is the end product of glycolysis. It represents a crucial metabolic crossroads, as its fate depends on the availability of oxygen and the cell’s energy demands.
Ketone Oxidation: Ketone oxidation is the metabolic process where ketone bodies (acetoacetate and beta-hydroxybutyrate) are broken down to produce acetyl CoA, which can then enter the Krebs cycle for energy generation. This is an important alternative fuel source during prolonged fasting or starvation.
Ketogenic Amino Acids: Ketogenic amino acids are those whose carbon skeletons can be converted into acetyl CoA or acetoacetate, which can then be used to synthesize ketone bodies or fatty acids. Examples include leucine and lysine.
Ketogenesis: Ketogenesis is the biochemical process that produces ketone bodies from acetyl CoA, primarily in the liver, particularly during periods of low carbohydrate intake or prolonged fasting. These ketone bodies serve as an alternative fuel source for the brain and other tissues.
Triglycerides: Triglycerides are the main form of fat storage in the body, composed of a glycerol backbone esterified to three fatty acid chains. They represent a highly concentrated form of energy, storing more than twice the energy per gram compared to carbohydrates or proteins.
Fatty acids: Fatty acids are long hydrocarbon chains with a carboxyl group, serving as a major component of lipids and a crucial energy source. They are liberated from triglycerides and undergo beta-oxidation to produce acetyl CoA.
Lipolysis/Beta-Oxidation: Lipolysis is the breakdown of triglycerides into glycerol and fatty acids, while beta-oxidation is the metabolic process that breaks down fatty acids into acetyl CoA. These processes occur primarily in the mitochondria and are major sources of energy.
Lipogenesis: Lipogenesis is the metabolic process of synthesizing fatty acids from acetyl CoA, typically occurring when there is an excess of energy intake (e.g., carbohydrates). These newly synthesized fatty acids can then be stored as triglycerides.
Glucogenic Amino Acids: Glucogenic amino acids are those whose carbon skeletons can be converted into pyruvate or intermediates of the Krebs cycle, which can then be used to synthesize glucose through gluconeogenesis. Examples include alanine and aspartate.
Acetyl CoA: Acetyl coenzyme A (acetyl CoA) is a central molecule in metabolism, formed from the breakdown of carbohydrates, fats, and amino acids. It is the primary substrate that enters the Krebs cycle, linking various metabolic pathways.
Certain Amino Acids: These amino acids refer to those that can directly enter the Krebs cycle as intermediates, such as alpha-ketoglutarate, succinyl CoA, or oxaloacetate. Their catabolism directly contributes to the cycle’s function and energy production.
Krebs cycle: The Krebs cycle, also known as the citric acid cycle, is a central metabolic pathway in aerobic organisms that oxidizes acetyl CoA, generating electron carriers (NADH and FADH2) and a small amount of ATP. It is a major hub for both catabolic and anabolic reactions.
Electron transport chain: The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane, responsible for the vast majority of ATP production during aerobic respiration. Electrons from NADH and FADH2 are passed along the chain, creating a proton gradient that drives ATP synthesis.
H2O – 2H+ + O2: This represents the final step of the electron transport chain, where oxygen acts as the final electron acceptor, combining with electrons and protons to form water. This reaction is crucial for continuously regenerating NAD+ and FAD, allowing the Krebs cycle and glycolysis to proceed.
The human body is a marvel of biochemical engineering, constantly balancing the breakdown of complex molecules (catabolism) with the synthesis of new ones (anabolism) to sustain life. This intricate interplay, collectively known as metabolism, ensures a continuous supply of energy and the necessary building blocks for growth, repair, and maintenance. From the moment we ingest food, nutrients embark on a complex journey through various metabolic pathways, ultimately contributing to either energy production or storage. Understanding this dynamic balance is fundamental to comprehending human health, disease, and the efficacy of different dietary approaches.
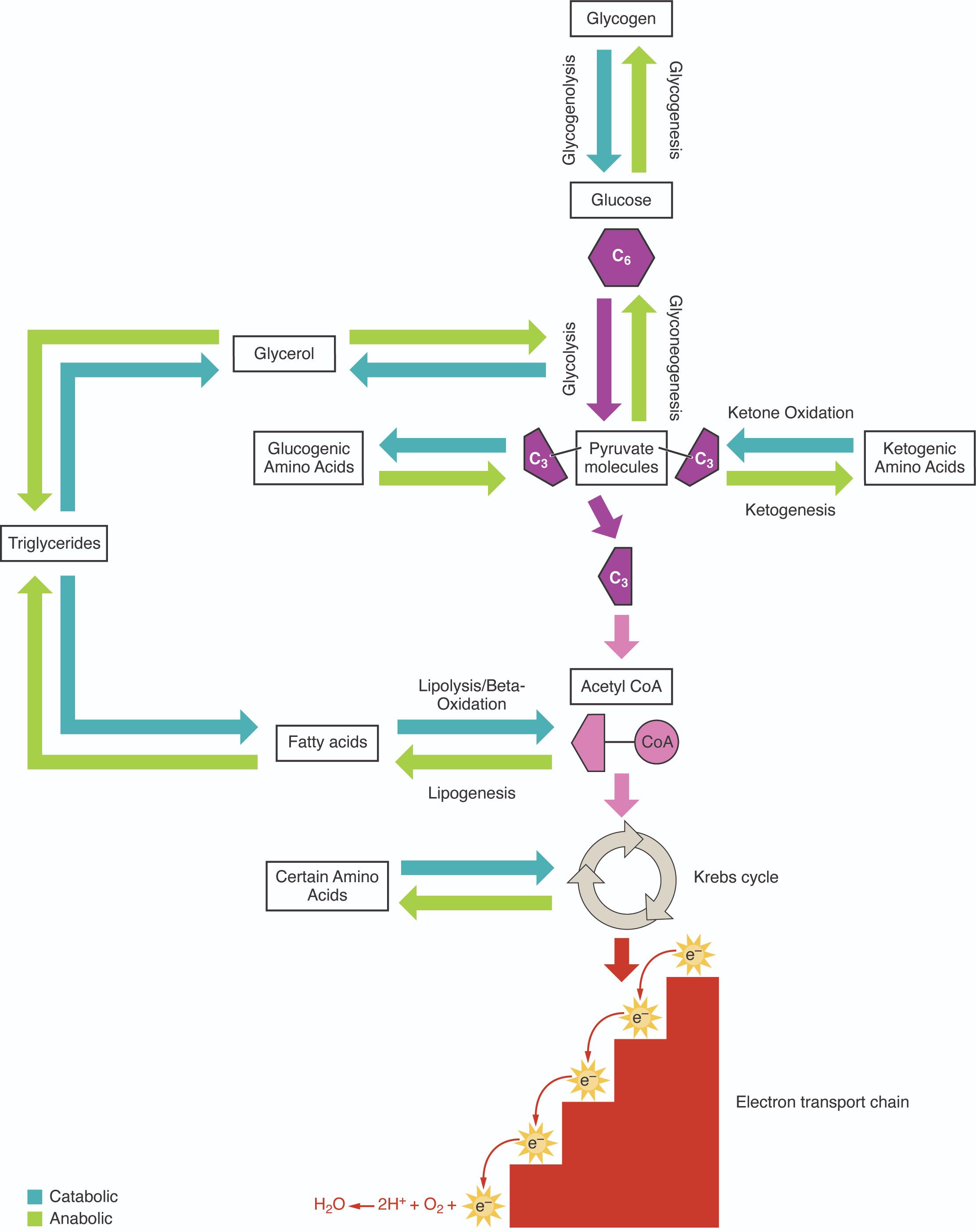
The diagram above beautifully illustrates the interconnectedness of these catabolic and anabolic pathways, showcasing how major macronutrients—carbohydrates, fats, and proteins—are metabolized. Carbohydrates, primarily in the form of glucose, are a readily available energy source. Glucose can be broken down through glycolysis, or stored as glycogen via glycogenesis. Conversely, when energy is needed, glycogen can be mobilized through glycogenolysis, and glucose can be synthesized from non-carbohydrate precursors via gluconeogenesis. These reversible pathways highlight the body’s remarkable flexibility in managing glucose homeostasis.
Fats, stored as triglycerides, represent a highly efficient energy reserve. During times of energy demand, triglycerides undergo lipolysis, releasing glycerol and fatty acids. Fatty acids are then broken down through beta-oxidation to produce acetyl CoA, which can enter the Krebs cycle. Glycerol, being glucogenic, can be converted to pyruvate or glucose. Proteins, composed of amino acids, also contribute to energy metabolism, particularly when carbohydrate and fat stores are depleted. Amino acids are categorized as either glucogenic (feeding into glucose production) or ketogenic (forming ketone bodies or fatty acids), demonstrating their diverse roles.
- Catabolism releases energy, while anabolism consumes it.
- Glucose is the primary energy source, stored as glycogen.
- Fats are efficient energy reserves, broken down into fatty acids and glycerol.
- Amino acids can be glucogenic or ketogenic, contributing to both energy and glucose production.
At the heart of aerobic energy production lies the Krebs cycle and the electron transport chain. Acetyl CoA, derived from the breakdown of glucose, fatty acids, and ketogenic amino acids, feeds into the Krebs cycle. This cyclical pathway systematically oxidizes acetyl CoA, generating reduced electron carriers (NADH and FADH2) and a small amount of ATP. These electron carriers then donate their electrons to the electron transport chain, a series of protein complexes embedded in the inner mitochondrial membrane.
The electron transport chain is where the vast majority of ATP is generated through oxidative phosphorylation. As electrons move down the chain, energy is released and used to pump protons across the mitochondrial membrane, creating an electrochemical gradient. This gradient drives the synthesis of ATP, effectively converting the energy stored in nutrient molecules into a usable form for the cell. Oxygen plays a critical role as the final electron acceptor in this process, combining with electrons and protons to form water.
Imbalances in these delicate metabolic pathways can lead to various medical conditions. For instance, uncontrolled diabetes mellitus is characterized by impaired glucose metabolism, affecting both glycolysis and gluconeogenesis, and often leading to increased reliance on fat and protein breakdown for energy, which can result in ketogenesis. Similarly, genetic disorders affecting specific enzymes in these pathways can disrupt nutrient processing, leading to the accumulation of toxic intermediates or severe energy deficits. Understanding this intricate metabolic map is paramount for diagnosing and treating such complex conditions effectively.
In conclusion, the constant interplay between catabolic and anabolic pathways is the bedrock of cellular function and overall human health. From the breakdown of glucose and fats to the versatile roles of amino acids, every nutrient is carefully processed to either generate energy or build essential molecules. The efficiency of the Krebs cycle and the electron transport chain in extracting energy is paramount for sustaining life. This profound understanding of metabolic interconnections not only illuminates the complexity of our internal systems but also provides critical insights for managing health and disease.

