The image provided illustrates a bileaflet mechanical heart valve, a sophisticated prosthetic device widely used in cardiovascular surgery to replace diseased native heart valves. Engineered for maximum durability and hemodynamic efficiency, this valve is constructed primarily from robust materials like pyrolytic carbon. It functions by responding to pressure gradients within the heart, opening to permit forward blood flow and closing to prevent backflow. Due to its superior design compared to earlier generations of mechanical valves, the bileaflet model has become the standard of care for patients requiring a long-lasting valvular replacement.
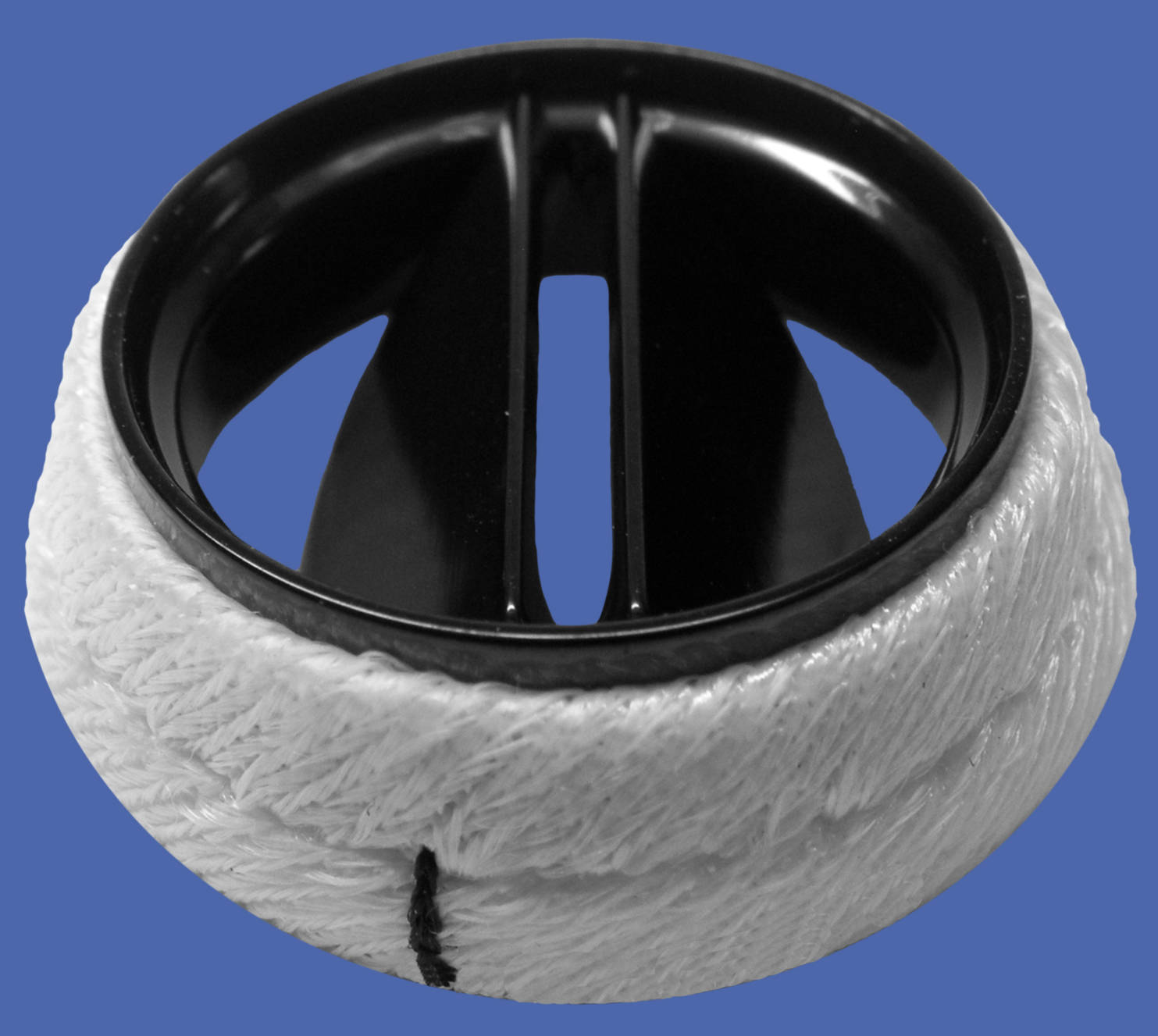
Sewing Ring: This white, fabric-like cuff surrounds the outer perimeter of the valve and is typically manufactured from biocompatible materials such as woven Dacron or Teflon. It acts as the interface between the prosthetic device and the patient’s heart tissue, providing a sturdy margin through which the surgeon passes sutures to anchor the valve securely in place.
Valve Housing: The rigid, black circular frame serves as the stent or body of the prosthesis, maintaining the shape of the orifice. Usually made from pyrolytic carbon, the housing supports the hinge mechanism and ensures that the valve maintains its structural integrity against the high-pressure environment of the cardiac cycle.
Leaflets: These are the two semi-circular occluder discs located inside the housing that pivot on hinges to control blood flow. They open to a near-vertical position to allow blood to pass through a central and two side orifices, and they pivot closed to seal the valve and prevent regurgitation.
Evolution and Mechanics of the Bileaflet Design
The bileaflet mechanical valve represents a significant leap forward in cardiac device engineering, improving upon earlier “ball-and-cage” and “tilting-disc” designs. Introduced extensively in the late 1970s, this design features two semicircular leaflets that pivot on hinges. When the heart contracts (systole) or relaxes (diastole), depending on the valve’s position, the pressure differential forces the leaflets open. Unlike older models that obstructed central flow, the bileaflet design allows for a central flow pattern, which significantly reduces turbulence and the pressure gradient across the valve.
One of the defining features of this valve is its construction from pyrolytic carbon. This material is exceptionally hard, resistant to fatigue, and chemically inert, making it ideal for a device that must open and close approximately 40 million times per year. While biological tissue valves may degenerate over 10 to 15 years, a mechanical bileaflet valve is designed to last the patient’s entire lifetime without structural failure. This makes it the preferred choice for younger patients who would otherwise require multiple re-operations if treated with tissue valves.
Despite its mechanical superiority, the interaction between blood and the artificial surface requires careful medical management. The hinge points and the carbon surface can induce clot formation if the blood is not adequately anticoagulated. Therefore, the physiological management of a patient with this implant focuses heavily on preventing thromboembolism while maintaining effective hemodynamics.
- Key advantages of the bileaflet valve include:
- Low profile design, making it easier to implant in smaller aortic roots.
- Central flow dynamics that minimize blood turbulence.
- Exceptional structural durability suitable for lifelong use.
- Radiopacity, allowing the valve to be easily visualized on X-rays.
Clinical Indications: Valvular Heart Disease
The primary medical indication for implanting a bileaflet mechanical heart valve is severe symptomatic valvular heart disease, most commonly aortic stenosis or mitral regurgitation. In aortic stenosis, the native aortic valve becomes calcified and narrowed, obstructing blood flow from the left ventricle into the aorta. This obstruction forces the heart to generate higher pressures to pump blood, leading to left ventricular hypertrophy (thickening of the muscle). If left untreated, this condition can progress to heart failure, syncope, or sudden cardiac death. By replacing the calcified valve with a mechanical prosthesis, surgeons relieve the obstruction, normalizing the pressure gradient and allowing the heart muscle to recover.
In cases of mitral regurgitation, the valve leaflets fail to close properly, causing blood to leak backward into the left atrium during ventricular contraction. This volume overload forces the heart to dilate and work inefficiently, eventually causing pulmonary hypertension and congestive heart failure. The bileaflet valve restores valvular competence, ensuring unidirectional blood flow. However, the introduction of a mechanical surface poses a risk of thrombosis. Blood platelets can aggregate on the valve leaflets or hinges, potentially leading to a stroke.
To mitigate these risks, patients must adhere to lifelong anticoagulation therapy, typically with a Vitamin K antagonist like Warfarin. Physicians closely monitor the patient’s International Normalized Ratio (INR) to keep the blood within a therapeutic range—thin enough to prevent clots on the valve, but thick enough to prevent spontaneous bleeding. Furthermore, because mechanical valves destroy a small number of red blood cells due to shear stress (a condition known as hemolysis), patients are monitored for anemia. Despite these management requirements, the bileaflet valve remains a life-saving intervention for patients with advanced aortic stenosis or insufficiency.
Conclusion
The bileaflet mechanical valve stands as a testament to the advancements in biomedical engineering, offering a durable and hemodynamically efficient solution for complex cardiac pathologies. By mimicking the essential one-way function of the heart’s natural valves, it alleviates the immense strain caused by stenosis or regurgitation, significantly improving patient survival and quality of life. While the necessity for lifelong anticoagulation remains a critical consideration, the trade-off provides patients with a robust device capable of functioning flawlessly for decades.

