Fondaparinux is a synthetic pentasaccharide anticoagulant used primarily for the prevention and treatment of venous thromboembolism. The chemical structure depicted represents a specific sequence of five carbohydrate units designed to mimic the high-affinity binding site of natural heparin for Antithrombin III. By understanding the molecular arrangement of sulfate and carboxyl groups within this molecule, medical professionals can better grasp its precise mechanism of action, its selectivity for Factor Xa, and its distinct clinical advantages over traditional blood thinners.
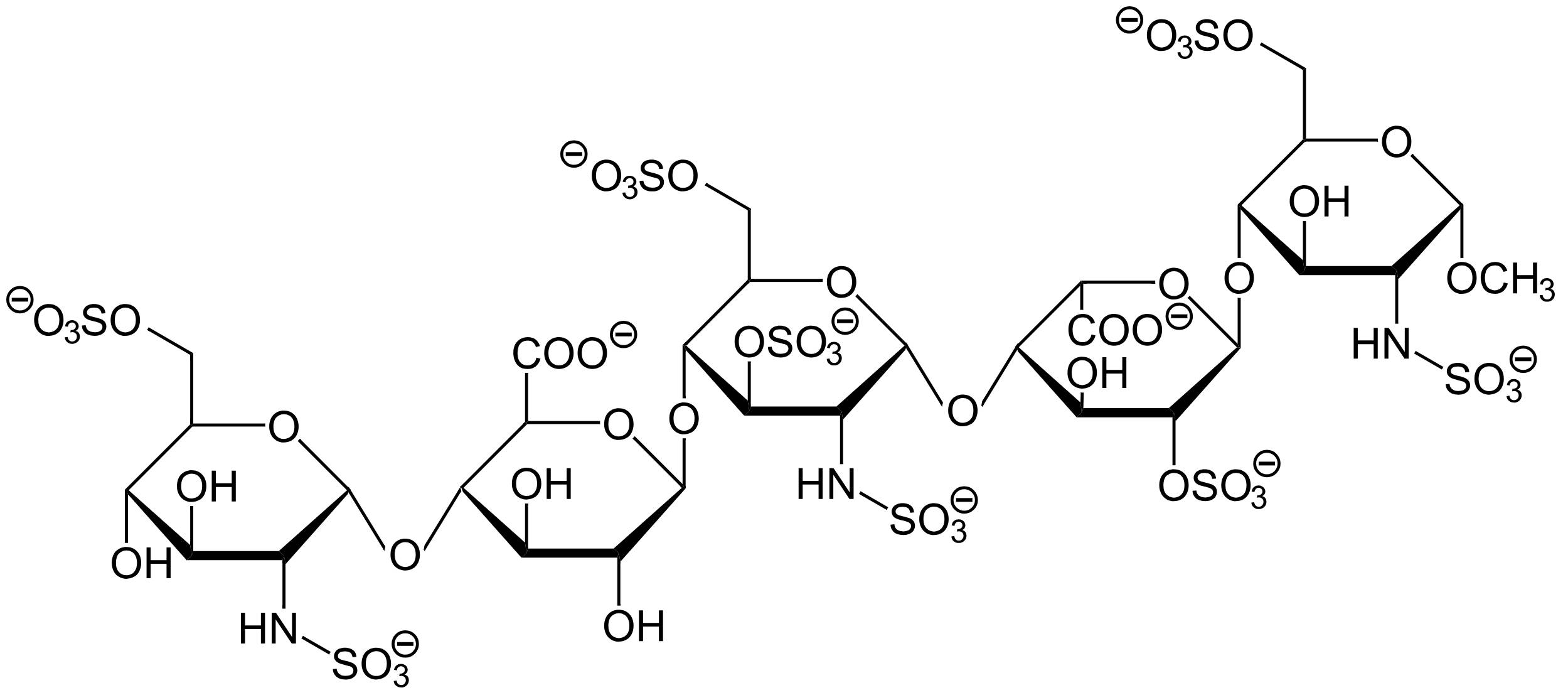
Chemical Structure Breakdown
Sulfate Groups (-OSO3 and -NHSO3):
The diagram displays multiple sulfate groups attached to oxygen and nitrogen atoms on the sugar rings. These highly negatively charged groups are critical for the molecule’s biological activity, as they facilitate strong electrostatic interactions with the positively charged amino acid residues (such as lysine and arginine) on the Antithrombin III protein.
Carboxyl Groups (-COO):
Located on the uronic acid residues (the second and fourth rings from the left), these carboxyl groups contribute to the overall negative charge and polarity of the molecule. They play a supportive role in orienting the molecule correctly within the binding pocket of Antithrombin, ensuring a stable conformational change.
Monosaccharide Rings:
The backbone of Fondaparinux consists of five specific sugar rings (monosaccharides) linked together: D-glucosamine, D-glucuronic acid, and L-iduronic acid derivatives. This specific pentasaccharide sequence is the minimum structural unit required to bind Antithrombin with high affinity, a feature originally identified within larger polymeric heparin chains.
Glycosidic Linkages:
The sugar rings are connected by oxygen bridges known as glycosidic bonds. These bonds create a linear chain structure that is flexible enough to drape over the surface of the Antithrombin protein but rigid enough to maintain the specific spatial arrangement of the active sulfate and carboxyl groups.
O-Methyl Group (-OCH3):
Visible on the far-right sugar unit (the reducing end), the O-methyl group indicates that this is a synthetic molecule with a “capped” end. This chemical modification stabilizes the molecule and distinguishes it from natural enzymatic degradation products found in biological systems.
The Science of Synthetic Anticoagulation
Fondaparinux represents a significant milestone in medicinal chemistry as the first selective Factor Xa inhibitor synthesized in a laboratory. Unlike unfractionated heparin, which is a heterogeneous mixture of polysaccharide chains derived from animal tissues (typically porcine intestine or bovine lung), Fondaparinux is a chemically synthesized, single-entity drug. This synthetic nature ensures batch-to-batch consistency and eliminates the risk of viral contamination that can be associated with animal-derived products.
The primary pharmacological advantage of this molecule lies in its specificity. The pentasaccharide structure binds exclusively to Antithrombin III (ATIII). Once bound, it induces a permanent conformational change in ATIII, increasing its ability to neutralize Factor Xa by approximately 300 times. Importantly, because the chain length is relatively short (only five sugar units), it is too short to bridge Antithrombin to Thrombin (Factor IIa). Therefore, Fondaparinux strictly inhibits Factor Xa without directly affecting Thrombin, providing a targeted approach to interrupting the coagulation cascade.
Clinically, this drug is preferred in many scenarios because of its predictable pharmacokinetic profile. It has a long half-life of approximately 17 to 21 hours, allowing for once-daily subcutaneous administration. Furthermore, because it lacks the longer chains found in heparin that interact with Platelet Factor 4, it rarely causes Heparin-Induced Thrombocytopenia (HIT), a severe immune-mediated clotting disorder associated with traditional heparin therapy.
Key characteristics of Fondaparinux include:
- Mechanism: Indirect Factor Xa inhibition via Antithrombin activation.
- Origin: Fully synthetic manufacturing process.
- Bioavailability: 100% bioavailability after subcutaneous injection.
- Renal Clearance: Eliminated unchanged by the kidneys, requiring dose adjustments in patients with renal impairment.
Medical Application: Treating Venous Thromboembolism
The primary clinical indication for Fondaparinux is the management of Venous Thromboembolism (VTE), which encompasses both Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE). DVT is a condition where a blood clot forms in a deep vein, usually in the lower leg, thigh, or pelvis. The danger of DVT lies not only in the local obstruction of blood flow, which causes swelling and pain, but in the risk of the clot dislodging.
If a portion of the clot breaks free (embolizes), it travels through the venous system to the right side of the heart and into the lungs, causing a Pulmonary Embolism. This blockage in the pulmonary arteries can be life-threatening, leading to low oxygen levels, heart strain, and potential cardiac arrest. Conditions such as major orthopedic surgery (hip or knee replacement), abdominal surgery, and prolonged immobility significantly increase the risk of VTE.
By selectively inhibiting Factor Xa, the drug effectively prevents the generation of Thrombin, thereby stopping the conversion of fibrinogen to fibrin. This halts the growth of existing clots and prevents the formation of new ones. Because the coagulation cascade is an amplification process—where one molecule of Factor Xa can lead to the generation of over 1,000 molecules of Thrombin—inhibiting the cascade at the Factor Xa level is a highly efficient strategy for thrombosis prophylaxis and treatment.
Conclusion
The 2D skeletal structure of Fondaparinux highlights the precision of modern pharmacology, where specific chemical groups are engineered to achieve a targeted physiological response. By isolating and synthesizing the exact pentasaccharide sequence responsible for Antithrombin binding, scientists created a potent anticoagulant with a high safety profile. Understanding this structure helps explain why the drug is effective for preventing dangerous clotting events while minimizing risks associated with older, biological blood thinners.

