The body’s acid-base balance is a tightly regulated physiological process, with a normal blood pH range of 7.35 to 7.45. Deviations from this narrow window, resulting in either acidosis (pH below 7.35) or alkalosis (pH above 7.45), can profoundly affect multiple organ systems. These imbalances can arise from various underlying conditions, and their symptoms can range from subtle to life-threatening. Understanding the diverse clinical manifestations of acidosis and alkalosis is critical for timely diagnosis and appropriate medical intervention, often guided by comprehensive blood tests.
Deconstructing the Symptoms of Acid-Base Imbalances
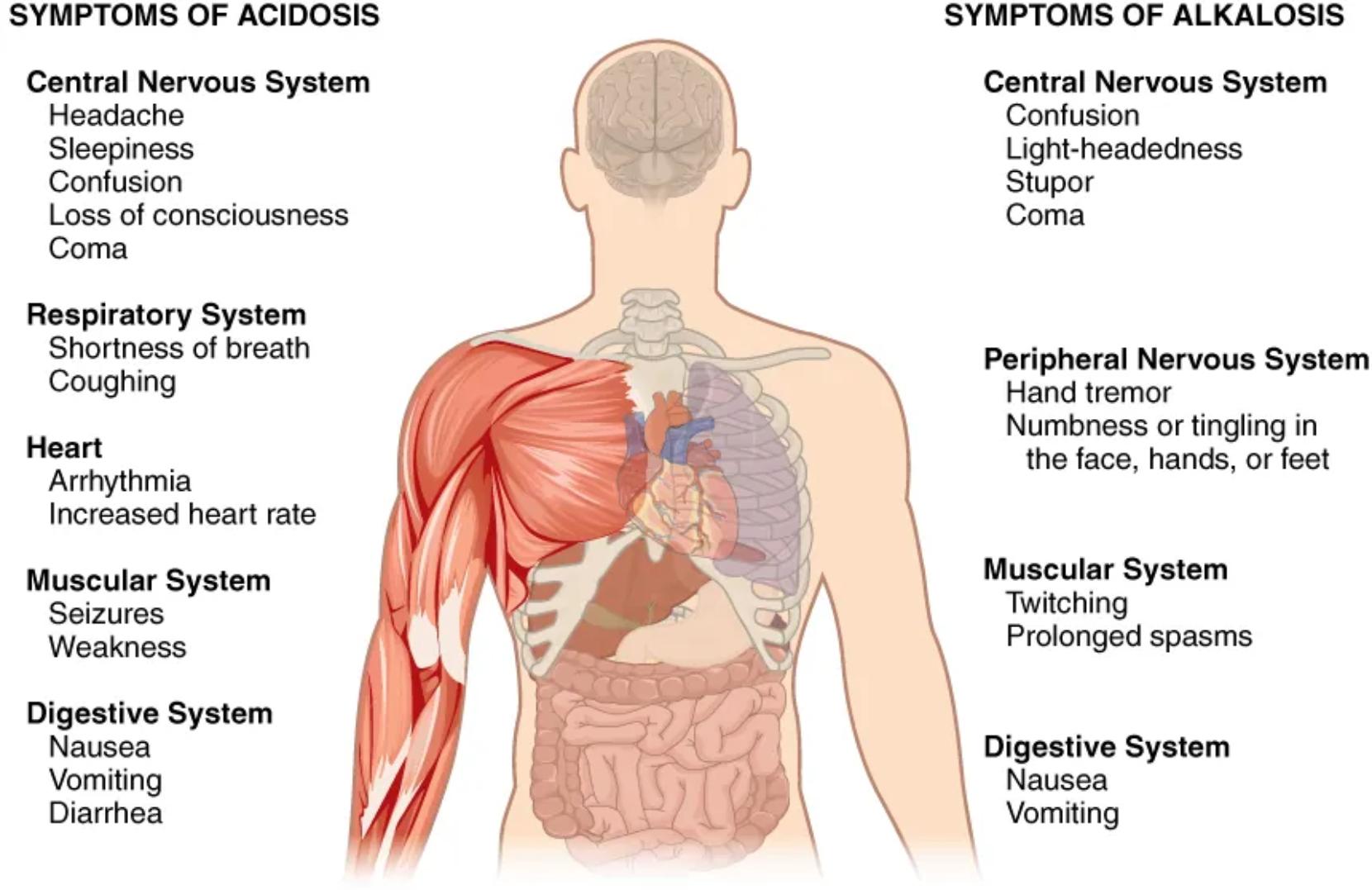
SYMPTOMS OF ACIDOSIS: This section outlines the various clinical signs and effects observed when the body’s blood pH is abnormally low (below 7.35). Acidosis signifies an excess of hydrogen ions, which disrupts cellular function across multiple organ systems. The symptoms listed are a direct consequence of this systemic cellular dysfunction.
Central Nervous System (Acidosis): The central nervous system is highly sensitive to pH changes. In acidosis, symptoms may include headache, sleepiness, and confusion, progressing to a loss of consciousness and even coma in severe cases. These neurological impairments reflect the disrupted metabolic activity and altered neurotransmission within the brain.
Headache (Acidosis): A common initial symptom of acidosis, often described as dull and persistent. It is believed to be due to cerebral vasodilation in response to increased CO2 levels in respiratory acidosis, or direct effects of acidosis on neuronal function.
Sleepiness (Acidosis): Refers to an unusual or excessive desire to sleep, often accompanied by lethargy and reduced mental alertness. This reflects a general depression of central nervous system activity due to the acidic environment.
Confusion (Acidosis): A state of disorientation, impaired judgment, and difficulty thinking clearly. It indicates a significant impact of acidosis on cognitive function and can worsen as the condition progresses.
Loss of consciousness (Acidosis): A more severe neurological symptom where an individual becomes unresponsive to external stimuli. This signifies a profound disturbance in brain function due to severe acidosis.
Coma (Acidosis): The most severe state of unconsciousness, where an individual is completely unresponsive. Coma in acidosis indicates a life-threatening level of acid-base imbalance that requires immediate medical intervention.
Respiratory System (Acidosis): The respiratory system often attempts to compensate for metabolic acidosis, leading to characteristic breathing patterns. However, respiratory acidosis itself can exacerbate these symptoms.
Shortness of breath (Acidosis): A subjective feeling of not getting enough air, also known as dyspnea. In acidosis, this can be part of the compensatory mechanism (Kussmaul breathing) to expel CO2, or a symptom of the underlying cause.
Coughing (Acidosis): While not exclusive to acidosis, coughing can be associated, particularly if the underlying cause involves respiratory compromise leading to respiratory acidosis.
Heart (Acidosis): The cardiovascular system is significantly affected by pH changes, with acidosis often leading to myocardial depression.
Arrhythmia (Acidosis): Irregular heart rhythms can occur due to acidosis altering cardiac cell membrane potentials and ion channel function. This can range from benign to life-threatening dysrhythmias.
Increased heart rate (Acidosis): Tachycardia is a common compensatory response to acidosis, as the heart tries to maintain cardiac output in the face of myocardial depression or to improve tissue oxygenation.
Muscular System (Acidosis): Acidosis can impair muscle function and excitability.
Seizures (Acidosis): In severe acidosis, especially if there’s significant electrolyte imbalance, the brain’s electrical activity can become unstable, leading to seizures.
Weakness (Acidosis): General muscle weakness and fatigue are common due to the direct effects of acidosis on muscle cell metabolism and contractility.
Digestive System (Acidosis): The gastrointestinal tract can also exhibit symptoms in acidosis.
Nausea (Acidosis): A feeling of sickness with an inclination to vomit. It is a common non-specific symptom associated with various systemic disturbances, including acidosis.
Vomiting (Acidosis): The involuntary expulsion of stomach contents. This can exacerbate electrolyte imbalances and fluid loss, further complicating the acid-base disorder.
Diarrhea (Acidosis): Frequent, loose, watery stools. This symptom can be both a cause (e.g., loss of bicarbonate) and a symptom of acidosis.
SYMPTOMS OF ALKALOSIS: This section details the various clinical signs and effects observed when the body’s blood pH is abnormally high (above 7.45). Alkalosis signifies a deficit of hydrogen ions, which also disrupts normal cellular processes and neurological function.
Central Nervous System (Alkalosis): Similar to acidosis, the central nervous system is highly vulnerable to alkaline conditions. Symptoms often involve altered consciousness and neurological excitability.
Confusion (Alkalosis): Disorientation and difficulty with clear thinking, similar to acidosis but stemming from different pathophysiological mechanisms related to increased neuronal excitability and altered cerebral blood flow in alkalosis.
Light-headedness (Alkalosis): A feeling of dizziness or faintness, often due to cerebral vasoconstriction in response to decreased CO2 levels in respiratory alkalosis, reducing cerebral blood flow.
Stupor (Alkalosis): A state of near-unconsciousness or partial unconsciousness, where an individual is only briefly aroused by strong stimuli. It is a more severe form of altered mental status than confusion.
Coma (Alkalosis): The most severe state of unconsciousness, indicating a profound and life-threatening acid-base imbalance due to severe alkalosis.
Peripheral Nervous System (Alkalosis): Alkalosis tends to increase neuronal excitability, leading to symptoms in the peripheral nervous system.
Hand tremor (Alkalosis): Involuntary rhythmic muscle contractions, often seen in the hands. This is due to increased neuromuscular irritability caused by alkalosis, particularly affecting calcium binding.
Numbness or tingling in the face, hands, or feet (Alkalosis): Known as paresthesias, these sensations are common in alkalosis. They result from increased nerve excitability and altered calcium ionization, leading to nerve hyperexcitability.
Muscular System (Alkalosis): Increased excitability extends to the muscular system.
Twitching (Alkalosis): Involuntary muscle contractions or fasciculations. This is a manifestation of increased neuromuscular irritability.
Prolonged spasms (Alkalosis): More severe and sustained muscle contractions, such as tetany, which can be quite painful and debilitating. This is a hallmark symptom of severe hypocalcemia induced by alkalosis.
Digestive System (Alkalosis): The gastrointestinal system can also be affected by alkalosis.
Nausea (Alkalosis): A feeling of sickness with an inclination to vomit. This is a general symptom of systemic upset and can occur in both acidosis and alkalosis.
Vomiting (Alkalosis): The involuntary expulsion of stomach contents. Severe vomiting can also be a cause of metabolic alkalosis due to loss of gastric acid.
The diagram vividly illustrates the systemic impact of acid-base imbalances, categorizing the diverse symptoms associated with acidosis and alkalosis across various organ systems. These conditions, characterized by abnormal blood pH levels, underscore the critical importance of a finely tuned physiological environment for optimal health. While a healthy body maintains blood pH within a narrow range of 7.35 to 7.45, many underlying diseases and physiological stressors can disrupt this delicate balance.
Acidosis, defined as a blood pH below 7.35, signifies an excess of hydrogen ions. It can result from an accumulation of acids (metabolic acidosis) or an inability to properly expel carbon dioxide (respiratory acidosis). The symptoms are widespread and can include:
- Central Nervous System: Headache, sleepiness, confusion, and in severe cases, loss of consciousness and coma.
- Respiratory System: Shortness of breath and coughing.
- Heart: Arrhythmia and increased heart rate.
- Muscular System: Seizures and generalized weakness.
- Digestive System: Nausea, vomiting, and diarrhea.
Conversely, alkalosis, characterized by a blood pH above 7.45, indicates a deficit of hydrogen ions. This can stem from an excessive loss of acids (metabolic alkalosis) or hyperventilation leading to too much carbon dioxide expulsion (respiratory alkalosis). Its symptoms often reflect increased neuromuscular excitability and altered cerebral blood flow:
- Central Nervous System: Confusion, light-headedness, stupor, and potentially coma.
- Peripheral Nervous System: Hand tremor, numbness, or tingling in the face, hands, or feet.
- Muscular System: Twitching and prolonged spasms.
- Digestive System: Nausea and vomiting.
Both acidosis and alkalosis are serious medical conditions that require prompt diagnosis and treatment. The information presented here highlights that while some symptoms like confusion, nausea, and vomiting can be common to both conditions, other manifestations, particularly those related to neurological and muscular excitability, tend to differentiate them. A definitive diagnosis always relies on laboratory blood tests, specifically arterial blood gas analysis, which measures pH, partial pressure of carbon dioxide (PCO2), and bicarbonate (HCO3-) levels to pinpoint the specific type and severity of the imbalance.
The Profound Systemic Effects of Acidosis and Alkalosis
The maintenance of a stable acid-base balance is paramount for human physiology, with the blood pH typically held within a very tight range of 7.35 to 7.45. Even slight deviations, leading to either acidosis (excess acidity) or alkalosis (excess alkalinity), can significantly impair cellular function and disrupt normal bodily processes. These imbalances are not diseases in themselves but rather manifestations of underlying physiological disturbances or pathological conditions. Understanding their diverse symptomology across various organ systems is crucial for recognizing these critical states and guiding appropriate diagnostic and therapeutic interventions.
Acidosis, characterized by a systemic increase in hydrogen ion concentration, can result from either an overproduction of metabolic acids (e.g., lactic acidosis, diabetic ketoacidosis) or a failure of the respiratory system to adequately remove carbon dioxide (respiratory acidosis). The consequences are widespread, affecting organ systems in a generalized depressive manner. The central nervous system is particularly vulnerable, exhibiting symptoms such as headache, increasing sleepiness, and profound confusion, which can progress to loss of consciousness and, ultimately, coma in severe cases. The cardiovascular system may show arrhythmias and an increased heart rate, attempting to compensate for compromised myocardial contractility. Muscular weakness and even seizures can occur due to the direct impact of acidity on muscle and nerve excitability. The digestive system often responds with non-specific symptoms like nausea, vomiting, and diarrhea, further exacerbating fluid and electrolyte imbalances.
Conversely, alkalosis, defined by a decrease in hydrogen ion concentration, can arise from excessive loss of acids (e.g., severe vomiting leading to metabolic alkalosis) or hyperventilation, which dramatically lowers blood carbon dioxide levels (respiratory alkalosis). The clinical picture of alkalosis often involves signs of increased neuromuscular irritability and altered cerebral blood flow. Central nervous system manifestations include confusion, light-headedness, stupor, and coma, reflecting the brain’s sensitivity to alkaline conditions and reduced cerebral blood flow due to vasoconstriction from low CO2. The peripheral nervous system can manifest symptoms such as a hand tremor and characteristic numbness or tingling sensations (paresthesias) in the face, hands, and feet, attributed to increased nerve excitability and alterations in calcium binding. The muscular system may exhibit involuntary twitching and severe, prolonged muscle spasms (tetany), which are also linked to enhanced neuromuscular irritability, often secondary to reduced ionized calcium levels in alkalotic states. Nausea and vomiting are also commonly observed in digestive system involvement.
Recognizing the distinct, yet sometimes overlapping, symptoms of acidosis and alkalosis is the first step towards intervention. While clinical presentation provides valuable clues, a definitive diagnosis relies on arterial blood gas (ABG) analysis. This diagnostic tool provides precise measurements of blood pH, partial pressure of carbon dioxide (PCO2), and bicarbonate (HCO3-) levels, allowing clinicians to accurately identify the specific type and severity of acid-base disturbance. Prompt identification and treatment of the underlying cause are essential to prevent the progression to life-threatening complications and restore physiological balance.
Conclusion
The comprehensive overview of symptoms associated with acidosis and alkalosis underscores the pervasive influence of pH balance on overall physiological function. From the profound neurological effects to the subtle muscular disturbances, these conditions can dramatically impact an individual’s well-being. The information presented here serves as a vital guide for recognizing the clinical signs of these critical acid-base imbalances. While symptoms provide crucial indicators, a precise diagnosis through blood tests is indispensable for initiating timely and targeted treatment, ultimately aiming to restore the delicate equilibrium that is fundamental to life.

