This article explores Patent Ductus Arteriosus (PDA), a common congenital heart defect, through the lens of an anatomical diagram illustrating its impact on blood flow. We will delve into the normal fetal circulation, the physiological changes that should occur at birth, and how the persistence of the ductus arteriosus leads to abnormal shunting of blood, impacting cardiovascular health in neonates.
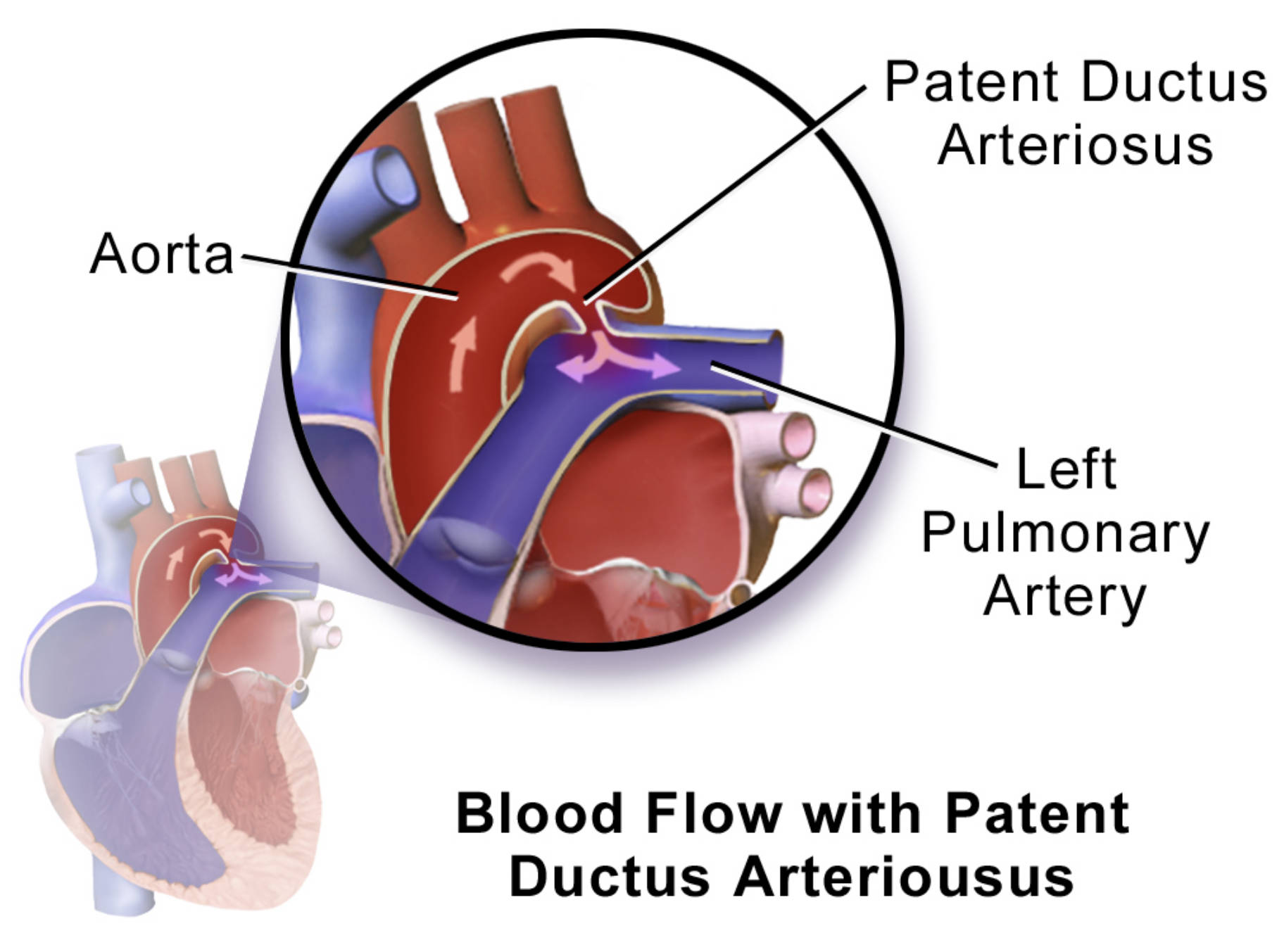
Patent Ductus Arteriosus: This refers to the failure of the ductus arteriosus to close shortly after birth. This persistent connection allows abnormal blood flow between the aorta and the pulmonary artery, bypassing the lungs.
Aorta: The body’s main artery, originating from the left ventricle of the heart and extending down to the abdomen. It distributes oxygenated blood to all parts of the body, except the lungs.
Left Pulmonary Artery: This artery carries deoxygenated blood from the right ventricle of the heart to the left lung. In the context of PDA, it receives abnormal shunted blood from the aorta.
Patent Ductus Arteriosus (PDA) is a congenital heart condition that arises when a crucial fetal blood vessel, the ductus arteriosus, fails to close shortly after birth. In the womb, this vessel plays a vital role in diverting blood away from the fetus’s developing lungs, which are not yet functional for gas exchange. However, after birth, as the lungs become active, the ductus arteriosus is supposed to constrict and close, typically within the first few days of life, becoming the ligamentum arteriosum. When this closure does not occur, it results in a persistent connection, leading to a unique pattern of abnormal blood flow.
The diagram effectively illustrates the altered hemodynamics associated with a PDA. Normally, the aorta carries oxygen-rich blood to the body, and the pulmonary artery carries deoxygenated blood to the lungs. With a PDA, oxygenated blood from the high-pressure aorta is shunted into the lower-pressure pulmonary artery, thereby increasing blood flow to the lungs. This left-to-right shunt is a defining characteristic of PDA and is central to its pathophysiology.
Understanding the implications of this abnormal shunting is essential for recognizing the signs, symptoms, and potential complications of PDA. The increased blood flow to the lungs can lead to various issues, depending on the size of the PDA and the amount of blood shunted. These can range from subtle findings to significant cardiovascular stress, especially in premature infants.
Fetal Circulation and Postnatal Transition
During fetal development, the circulatory system is uniquely adapted to life within the womb, where the placenta serves as the organ for gas exchange. The ductus arteriosus is a critical component of this system, acting as a shortcut that connects the pulmonary artery directly to the aorta. This allows the majority of blood from the right ventricle to bypass the non-functional fetal lungs and enter the systemic circulation. Only a small amount of blood flows through the pulmonary arteries to nourish the developing lung tissue.
At birth, a dramatic shift occurs in the circulatory system. As the newborn takes its first breath, the lungs expand, and pulmonary vascular resistance decreases significantly. This drop in resistance, coupled with an increase in systemic vascular resistance (due to the clamping of the umbilical cord), reverses the direction of blood flow through the ductus arteriosus. Instead of shunting blood away from the lungs, the pressure gradient now favors blood flow from the aorta to the pulmonary artery. The increase in oxygen tension and a decrease in prostaglandins (substances that keep the duct open) trigger the constriction and functional closure of the ductus arteriosus, typically within 24 to 72 hours. Anatomical closure, through fibrosis, usually follows within a few weeks. Failure of this intricate process to complete results in a Patent Ductus Arteriosus.
Pathophysiology of Patent Ductus Arteriosus
In a patient with a PDA, the persistent connection between the aorta and the pulmonary artery creates a continuous left-to-right shunt. This means that oxygen-rich blood from the high-pressure aorta flows back into the lower-pressure pulmonary artery, as clearly indicated by the purple arrows in the diagram. This recirculation of blood through the lungs leads to increased pulmonary blood flow, which in turn can cause several problems. The left side of the heart, particularly the left atrium and left ventricle, must work harder to pump the extra volume of blood, leading to volume overload and eventual chamber enlargement.
The increased blood flow and pressure in the pulmonary arteries can lead to pulmonary hypertension over time. This can cause remodeling of the pulmonary blood vessels, making them stiff and narrowed, and eventually lead to irreversible pulmonary vascular disease. In severe, untreated cases, particularly in large PDAs, this can even reverse the shunt direction, leading to Eisenmenger syndrome, where deoxygenated blood flows from the pulmonary artery into the aorta, causing cyanosis. Premature infants are particularly susceptible to PDA because their ductus arteriosus is less responsive to the signals that trigger closure, and they have higher levels of circulating prostaglandins. The severity of PDA symptoms and complications often correlates with the size of the ductus and the magnitude of the shunt.
Clinical Manifestations, Diagnosis, and Management
The clinical presentation of PDA varies widely depending on the size of the shunt and the patient’s age. In full-term infants with a small PDA, symptoms may be absent, and the condition might only be detected during a routine physical examination by the presence of a characteristic continuous “machinery-like” heart murmur. Larger PDAs, especially in premature infants, can lead to signs of heart failure, such as rapid breathing, poor feeding, poor weight gain, and increased susceptibility to respiratory infections. In severe cases, infants may develop signs of pulmonary hypertension.
Diagnosis of PDA is primarily made through a physical examination, followed by an echocardiogram. The echocardiogram provides real-time images of the heart and blood vessels, allowing for direct visualization of the PDA, assessment of its size, and quantification of the shunting blood flow. It also helps evaluate the impact on cardiac chambers and pulmonary artery pressures. Treatment options depend on the size of the PDA, the patient’s age, and the presence of symptoms. In premature infants, pharmacological closure using non-steroidal anti-inflammatory drugs (NSAIDs) like indomethacin or ibuprofen is often successful, as these medications inhibit prostaglandin synthesis, facilitating ductal constriction.
For larger PDAs or those that fail to close with medication, particularly in older infants and children, interventional procedures are often required. These can include catheter-based closure techniques, where a coil or device is deployed through a catheter to occlude the ductus, or surgical ligation, where the ductus is surgically tied off. The aim of treatment is to close the PDA, prevent long-term complications such as pulmonary hypertension and heart failure, and restore normal circulatory hemodynamics. Early diagnosis and appropriate management are crucial for ensuring optimal outcomes for individuals with Patent Ductus Arteriosus.

