Discover how your body expertly manages energy during the postabsorptive state, relying on stored glycogen, fats, and proteins to maintain blood glucose and cellular function. This article details the critical role of glucagon and its effects on the liver, muscle, and adipose tissue.
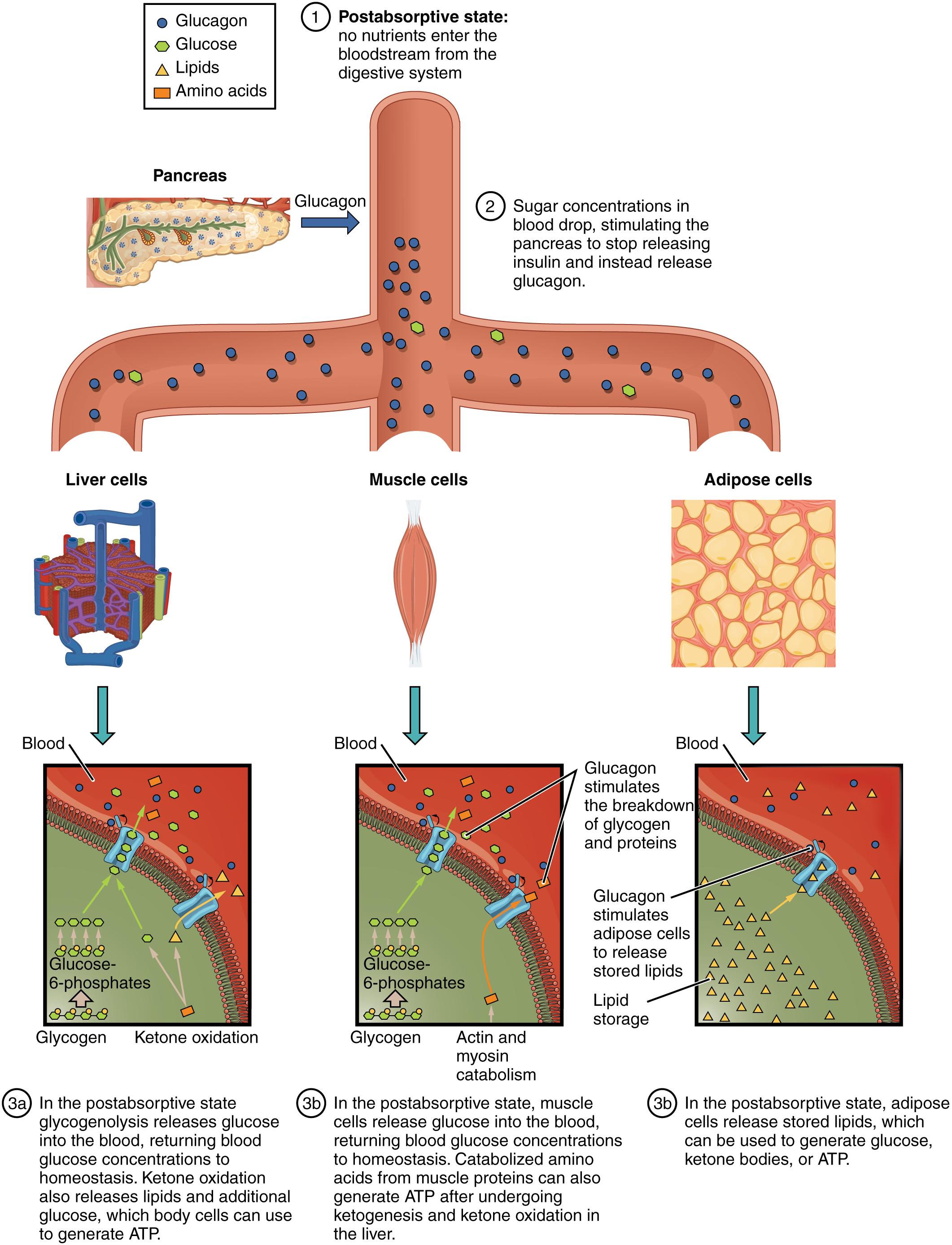
Pancreas: The pancreas is a vital endocrine and exocrine gland located in the abdomen, playing a central role in regulating blood glucose levels. Its alpha cells secrete glucagon, a hormone crucial for maintaining glucose homeostasis during periods of fasting.
Glucagon: Glucagon is a peptide hormone produced by the alpha cells of the pancreas, acting antagonistically to insulin. Its primary function is to raise blood glucose levels, particularly during the postabsorptive state, by stimulating glucose production and release from the liver.
Glycogen: Glycogen is a complex carbohydrate (polysaccharide) composed of many glucose units, serving as the primary storage form of glucose in animals, predominantly in the liver and muscles. It is rapidly broken down to release glucose when energy is needed.
Glucose: Glucose is a simple monosaccharide and the main source of energy for the body’s cells, especially the brain. Maintaining stable blood glucose levels is paramount for proper physiological function, and this is a key focus of the postabsorptive state.
Lipids: Lipids, including triglycerides and fatty acids, are a diverse group of organic compounds essential for long-term energy storage, cell membrane structure, and hormone production. During the postabsorptive state, stored lipids are mobilized to provide an alternative fuel source.
Amino acids: Amino acids are the fundamental building blocks of proteins, crucial for various bodily functions. In the postabsorptive state, amino acids can be catabolized (broken down) to provide energy or serve as precursors for glucose synthesis, especially in muscle tissue.
1 Postabsorptive state: no nutrients enter the bloodstream from the digestive system: This signifies the beginning of the postabsorptive, or fasting, state, where nutrient absorption from the digestive tract has ceased. The body must now shift its metabolic strategies to rely on its internal energy reserves to maintain blood glucose and fuel cellular activities.
2 Sugar concentrations in blood drop, stimulating the pancreas to stop releasing insulin and instead release glucagon.: As absorbed nutrients are utilized and glucose levels in the blood begin to fall, the stimulus for insulin release diminishes. Instead, the declining blood glucose activates the alpha cells of the pancreas to secrete glucagon, a hormone designed to counteract the drop in blood sugar.
Liver cells: Liver cells (hepatocytes) are central to metabolic regulation, especially during the postabsorptive state. They play a critical role in producing and releasing glucose into the bloodstream to maintain homeostasis.
Muscle cells: Muscle cells are significant energy consumers and also store glycogen, though their glycogen reserves are primarily for their own use. In the postabsorptive state, muscle proteins can be broken down to provide amino acids for gluconeogenesis in the liver.
Adipose cells: Adipose cells (adipocytes) are specialized for storing large quantities of energy in the form of triglycerides (fat). During the postabsorptive state, these cells become active in releasing fatty acids and glycerol to provide alternative fuel sources for other tissues.
3a In the postabsorptive state, liver glycogenolysis releases glucose into the blood, returning blood glucose concentrations to homeostasis. Ketone oxidation also releases lipids and additional glucose, which body cells can use to generate ATP.: The liver responds to glucagon by breaking down its stored glycogen (glycogenolysis) and releasing glucose directly into the bloodstream. Furthermore, it initiates gluconeogenesis and ketogenesis, providing alternative fuels like ketone bodies and additional glucose for cells, particularly the brain.
3b In the postabsorptive state, muscle cells release glucose into the blood, returning blood glucose concentrations to homeostasis. Catabolized amino acids from muscle proteins can also generate ATP after undergoing ketogenesis and ketone oxidation in the liver.: During fasting, muscle glycogen is primarily used for the muscle’s own energy needs. However, muscle proteins can be broken down into amino acids, which are then released into the bloodstream and transported to the liver. There, these amino acids can be used for gluconeogenesis, contributing to the maintenance of blood glucose levels.
3c In the postabsorptive state, adipose cells release stored lipids, which can be used to generate glucose, ketone bodies, or ATP.: Adipose tissue responds to glucagon by breaking down stored triglycerides into fatty acids and glycerol. These components are then released into the bloodstream, where fatty acids can be directly used as fuel by many tissues, and glycerol can be converted into glucose by the liver.
Glucose-6-phosphates: Glucose-6-phosphate is a critical intermediate in glucose metabolism, formed when glucose is phosphorylated upon entry into a cell. In the liver, it can be dephosphorylated to release free glucose into the blood.
Ketone oxidation: Ketone oxidation is the metabolic process where ketone bodies are broken down into acetyl CoA, which then enters the Krebs cycle to produce ATP. This is an important energy source for many tissues, especially the brain, during prolonged fasting.
Actin and myosin catabolism: Actin and myosin are the primary contractile proteins in muscle fibers. During prolonged fasting, these proteins can be broken down (catabolism) to release amino acids, which are then transported to the liver for gluconeogenesis, contributing to blood glucose maintenance.
Lipid storage: Lipid storage refers to the accumulation of fats, primarily triglycerides, in adipose tissue. In the postabsorptive state, the process reverses, with stored lipids being mobilized to provide energy.
The postabsorptive state, commonly known as the fasting state, is a physiological period that occurs roughly 4 hours after a meal, when nutrient absorption from the gastrointestinal tract has ceased. During this crucial phase, the body shifts its metabolic strategies from storing energy to mobilizing its internal reserves to maintain blood glucose homeostasis and provide fuel for all tissues, particularly the brain, which relies heavily on glucose. This intricate balancing act is primarily orchestrated by hormonal signals, ensuring a continuous supply of energy even in the absence of exogenous nutrient intake.
The decline in blood glucose concentrations during the postabsorptive state is the primary trigger for the pancreas to cease insulin secretion and instead release glucagon. Glucagon, a powerful catabolic hormone, acts antagonistically to insulin, initiating a cascade of events designed to raise blood glucose levels. This includes stimulating the breakdown of stored glycogen, promoting the synthesis of new glucose, and mobilizing alternative fuel sources like fatty acids. The precise regulation of glucagon secretion is vital for preventing hypoglycemia, a dangerously low blood glucose level.
This detailed diagram illustrates how the body strategically taps into its stored energy reserves during the postabsorptive state. The liver, muscle cells, and adipose tissue each play distinct yet complementary roles in this process. The coordinated action of these organs, primarily under the influence of glucagon, ensures that the body’s energy demands are met and that vital organs continue to function optimally, even when no new nutrients are coming in from digestion.
- The postabsorptive state occurs when nutrient absorption has stopped.
- Glucagon is the primary hormone, raising blood glucose.
- The body relies on stored glycogen, fats, and proteins.
- The liver, muscles, and adipose tissue mobilize energy reserves.
In the liver, glucagon rapidly stimulates glycogenolysis, the breakdown of stored glycogen into glucose, which is then released directly into the bloodstream. This is the body’s immediate response to declining blood glucose. As glycogen stores become depleted, the liver also initiates gluconeogenesis, synthesizing new glucose from non-carbohydrate precursors like amino acids (derived from muscle protein breakdown) and glycerol (from fat breakdown). Additionally, the liver ramps up ketogenesis, producing ketone bodies from fatty acids, which serve as an important alternative fuel source for many tissues, including the brain during prolonged fasting.
Muscle cells, while storing a significant amount of glycogen, primarily use these reserves for their own energy needs during activity. However, in the postabsorptive state, if fasting is prolonged, muscle proteins can be broken down (catabolized) into amino acids. These amino acids are then released into the bloodstream and transported to the liver, where they become substrates for gluconeogenesis, further contributing to maintaining blood glucose levels. This highlights the body’s willingness to sacrifice protein for glucose when energy resources are scarce.
Adipose tissue, the body’s primary long-term energy reservoir, becomes highly active in the postabsorptive state. Glucagon stimulates lipolysis, the breakdown of stored triglycerides into fatty acids and glycerol. These fatty acids are released into the bloodstream and can be directly utilized as fuel by many tissues, sparing glucose for the brain. The glycerol component is transported to the liver, where it can be converted into glucose via gluconeogenesis. This mobilization of fat reserves is crucial for providing a sustained energy supply during periods between meals.
In conclusion, the postabsorptive state represents a sophisticated metabolic adaptation, ensuring the continuous supply of energy and the maintenance of blood glucose homeostasis in the absence of nutrient intake. Through the coordinated actions of glucagon on the liver, muscle, and adipose tissue, the body efficiently mobilizes stored glycogen, fats, and even proteins to meet its energy demands. A comprehensive understanding of these physiological responses is vital not only for appreciating the body’s resilience but also for addressing metabolic imbalances seen in conditions such as hypoglycemia and various forms of metabolic stress.

