Stroke volume, the amount of blood pumped by the heart with each beat, is a critical determinant of cardiac output and overall cardiovascular health. This vital parameter is influenced by preload, contractility, and afterload, each shaped by a variety of physiological and environmental factors. Exploring these elements provides a deeper understanding of how the heart adapts to maintain efficient circulation under diverse conditions.
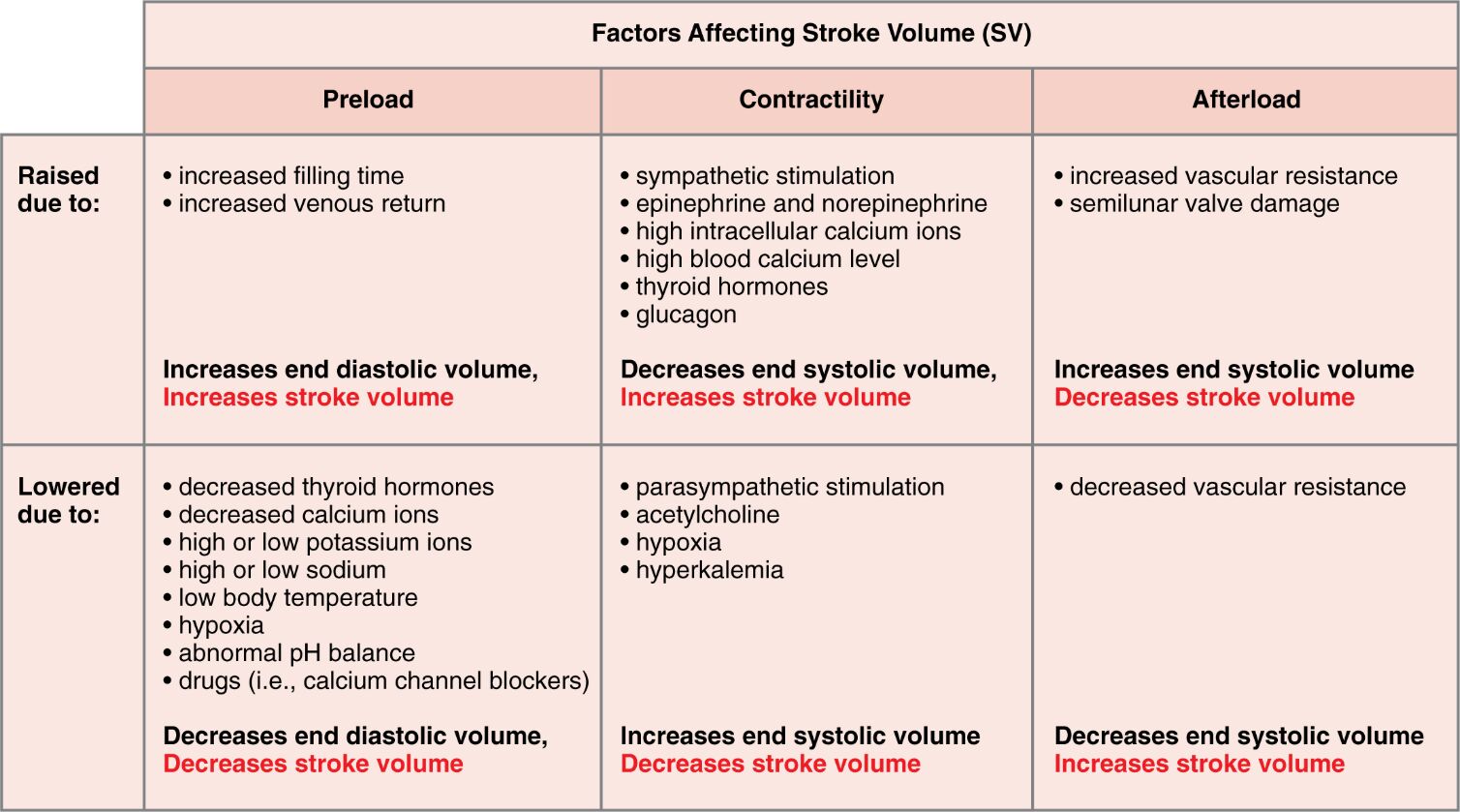
Preload Preload refers to the initial stretching of the cardiac myocytes prior to contraction, primarily determined by end-diastolic volume. Increased preload enhances stroke volume through the Frank-Starling mechanism, while decreased preload reduces it by limiting ventricular filling.
Contractility Contractility is the intrinsic ability of the heart muscle to contract, influencing the force of ejection and thus stroke volume. Enhanced contractility decreases end-systolic volume, increasing stroke volume, whereas reduced contractility has the opposite effect.
Afterload Afterload represents the resistance the heart must overcome to eject blood, affected by vascular resistance and valve function. Elevated afterload increases end-systolic volume and decreases stroke volume, while lowered afterload reduces end-systolic volume, enhancing stroke volume.
Raised due to: Raised due to: indicates conditions or factors that elevate preload, contractility, or afterload, impacting stroke volume. These changes can stem from physiological responses or pathological states, altering cardiac performance.
Lowered due to: Lowered due to: highlights factors that decrease preload, contractility, or afterload, influencing stroke volume in the opposite direction. These conditions can arise from metabolic imbalances or therapeutic interventions, affecting heart efficiency.
Increased filling time Increased filling time allows more blood to enter the ventricles, raising preload and subsequently increasing stroke volume. This occurs during slower heart rates, optimizing ventricular filling.
Increased venous return Increased venous return boosts preload by delivering more blood to the heart, enhancing stroke volume via greater ventricular stretch. This is common during exercise when skeletal muscle pumps improve circulation.
Sympathetic stimulation Sympathetic stimulation enhances contractility by releasing norepinephrine and epinephrine, increasing stroke volume through stronger contractions. This response prepares the heart for increased demand during stress or activity.
Epinephrine and norepinephrine Epinephrine and norepinephrine are catecholamines that boost contractility, reducing end-systolic volume and increasing stroke volume. These hormones are released by the adrenal glands during the fight-or-flight response.
High intracellular calcium ions High intracellular calcium ions strengthen contractility by enhancing myocardial contraction, leading to a lower end-systolic volume and higher stroke volume. This increase supports cardiac output during heightened activity.
High blood calcium level High blood calcium level improves contractility by facilitating calcium availability for muscle contraction, increasing stroke volume. This condition can result from dietary intake or hormonal regulation.
Thyroid hormones Thyroid hormones, such as T3 and T4, enhance contractility by boosting metabolic rate and cardiac muscle performance, increasing stroke volume over time. These hormones play a long-term role in cardiovascular function.
Glucagon Glucagon supports contractility by increasing cyclic AMP levels, enhancing myocardial contractility and thus stroke volume. This hormone acts as a secondary regulator during metabolic stress.
Increased vascular resistance Increased vascular resistance raises afterload, increasing end-systolic volume and decreasing stroke volume due to greater opposition to ejection. This can occur in conditions like hypertension.
Semilunar valve damage Semilunar valve damage elevates afterload by impeding blood flow, increasing end-systolic volume and reducing stroke volume. This damage can lead to regurgitation, affecting cardiac efficiency.
Decreased thyroid hormones Decreased thyroid hormones lower contractility by reducing metabolic support, decreasing stroke volume due to weaker contractions. This can occur in hypothyroidism, impacting heart performance.
Decreased calcium ions Decreased calcium ions reduce contractility by limiting muscle contraction strength, increasing end-systolic volume and decreasing stroke volume. This imbalance can arise from dietary deficiency or medication effects.
High or low potassium ions High or low potassium ions disrupt contractility by altering membrane potential, increasing end-systolic volume and decreasing stroke volume. These imbalances can lead to arrhythmias if severe.
High or low sodium ions High or low sodium ions affect contractility by influencing ion gradients, increasing end-systolic volume and decreasing stroke volume. This disruption can impair normal cardiac function.
Low body temperature Low body temperature reduces contractility by slowing metabolic processes, increasing end-systolic volume and decreasing stroke volume. This can occur during hypothermia, affecting heart rate and output.
Hypoxia Hypoxia lowers contractility by reducing oxygen availability, increasing end-systolic volume and decreasing stroke volume. This condition stresses the heart, limiting its pumping ability.
Abnormal pH balance Abnormal pH balance impairs contractility by disrupting cellular function, increasing end-systolic volume and decreasing stroke volume. Acidosis or alkalosis can both negatively impact the heart.
Drugs (i.e., calcium channel blockers) Drugs (i.e., calcium channel blockers) decrease contractility by inhibiting calcium entry, increasing end-systolic volume and decreasing stroke volume. These medications are used to manage hypertension or arrhythmias.
Parasympathetic stimulation Parasympathetic stimulation reduces contractility via acetylcholine release, increasing end-systolic volume and decreasing stroke volume. This effect predominates during rest to conserve energy.
Acetylcholine Acetylcholine lowers contractility by activating muscarinic receptors, increasing end-systolic volume and decreasing stroke volume. This neurotransmitter is key to parasympathetic control.
Hyperkalemia Hyperkalemia diminishes contractility by altering potassium levels, increasing end-systolic volume and decreasing stroke volume. This condition can lead to serious cardiac complications.
Decreased vascular resistance Decreased vascular resistance lowers afterload, decreasing end-systolic volume and increasing stroke volume by easing ejection. This can occur with vasodilation therapies.
Understanding Stroke Volume Basics
Stroke volume is a fundamental aspect of cardiac performance, reflecting the heart’s efficiency per beat. It adapts to physiological demands through the interplay of preload, contractility, and afterload.
- Preload determines the initial stretch of the ventricles, influencing filling volume.
- Contractility reflects the heart muscle’s contractile strength, driven by ion availability.
- Afterload represents the resistance faced during ejection, affecting output.
- These factors collectively determine stroke volume, impacting cardiac output.
- Hormonal influences, like thyroid hormones, modulate these parameters over time.
The Role of Preload in Stroke Volume
Preload is a key driver of stroke volume, shaped by filling dynamics. It ensures the heart can respond to varying blood return levels.
- Increased filling time allows greater ventricular filling, boosting stroke volume.
- Increased venous return enhances preload, leveraging the Frank-Starling mechanism.
- Reduced preload from low blood volume decreases stroke volume.
- Exercise increases venous return, optimizing preload and output.
- Dehydration can lower preload, reducing cardiac efficiency.
Impact of Raised and Lowered Preload
Changes in preload significantly alter stroke volume based on filling conditions. These shifts reflect the heart’s adaptability to circulatory needs.
- Raised due to: increased filling time supports higher stroke volume.
- Raised due to: enhanced venous return stretches the myocardium effectively.
- Lowered due to: decreased thyroid hormones reduce metabolic support.
- Lowered due to: hypoxia limits oxygen for optimal filling.
- Lowered due to: drugs (i.e., calcium channel blockers) impair filling dynamics.
Contractility and Its Effects
Contractility determines how forcefully the heart pumps, directly affecting stroke volume. It responds to neural and hormonal signals for optimal performance.
- Sympathetic stimulation increases contractility, enhancing stroke volume.
- Epinephrine and norepinephrine boost contractility during stress.
- High intracellular calcium ions strengthen contractility for better ejection.
- Parasympathetic stimulation reduces contractility, lowering stroke volume.
- Hyperkalemia disrupts contractility, impacting cardiac output.
Factors Raising and Lowering Contractility
Variations in contractility reflect the heart’s response to internal conditions. These changes ensure adaptability to physiological states.
- Raised due to: thyroid hormones enhance metabolic rate and contractility.
- Raised due to: glucagon supports contractility during stress.
- Lowered due to: acetylcholine dampens contractility at rest.
- Lowered due to: low body temperature slows contractility.
- Lowered due to: abnormal pH balance impairs contractility.
Afterload’s Influence on Stroke Volume
Afterload challenges the heart’s ejection phase, influencing stroke volume. It adjusts based on vascular conditions and valve integrity.
- Increased vascular resistance raises afterload, reducing stroke volume.
- Semilunar valve damage increases afterload, hindering ejection.
- Decreased vascular resistance lowers afterload, boosting stroke volume.
- High blood pressure elevates afterload, straining the heart.
- Vasodilators reduce afterload, improving cardiac output.
Raised and Lowered Afterload Dynamics
Fluctuations in afterload directly impact stroke volume through ejection resistance. These changes reflect cardiovascular health status.
- Raised due to: increased vascular resistance challenges ejection.
- Raised due to: semilunar valve damage obstructs blood flow.
- Lowered due to: decreased vascular resistance eases ejection.
- Low afterload supports higher stroke volume in healthy states.
- Pathological afterload increases can lead to heart failure risks.
In conclusion, stroke volume is a dynamic measure shaped by preload, contractility, and afterload, each influenced by a range of factors. Mastering these relationships enhances our ability to support cardiovascular health and address potential inefficiencies. Continued exploration of these mechanisms promises to improve diagnostic and therapeutic strategies.

