Explore ketogenesis, the crucial metabolic pathway by which the liver produces ketone bodies from excess acetyl CoA, providing an alternative fuel source for the brain and other tissues during periods of fasting or low carbohydrate intake. This process is essential for survival when glucose is scarce, demonstrating the body’s remarkable metabolic flexibility.
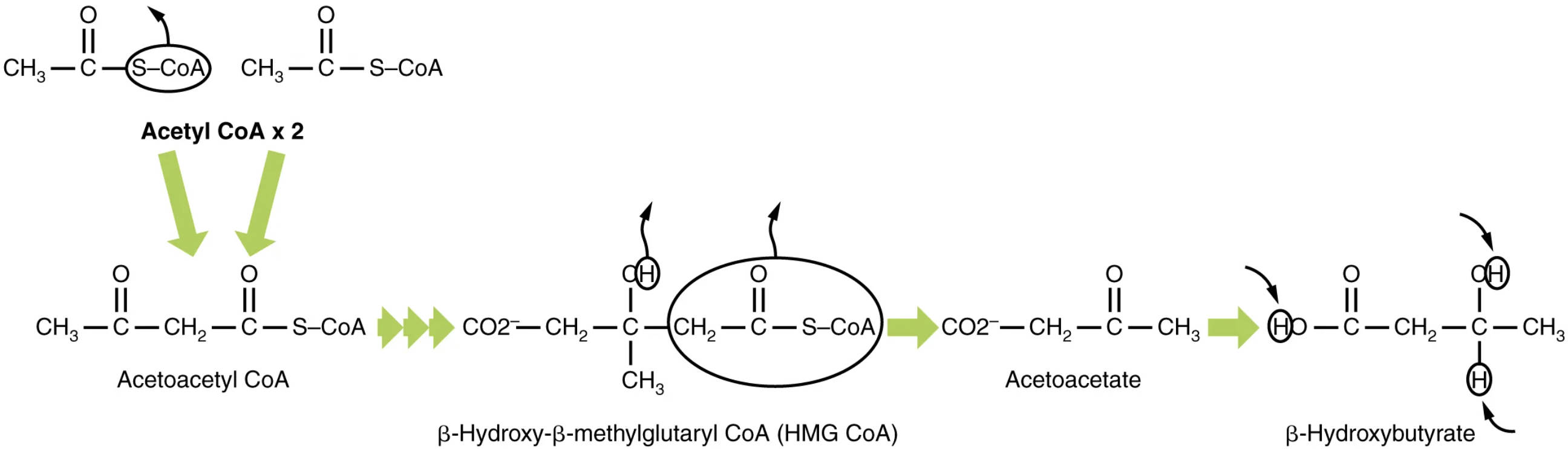
Understanding the Ketogenesis Diagram
Acetyl CoA x 2: This represents two molecules of acetyl coenzyme A, which serve as the primary building blocks for ketogenesis. Acetyl CoA is derived from the breakdown of fatty acids (beta-oxidation) and, to a lesser extent, from pyruvate and certain amino acids.
Acetoacetyl CoA: This four-carbon molecule is the first intermediate formed in ketogenesis, resulting from the condensation of two acetyl CoA molecules. It is a key precursor to the other ketone bodies and also an intermediate in fatty acid synthesis.
β-Hydroxy-β-methylglutaryl CoA (HMG CoA): This six-carbon intermediate is formed by the condensation of acetoacetyl CoA with a third molecule of acetyl CoA. HMG CoA is a crucial branch point in metabolism, as it can be directed towards either ketone body synthesis or cholesterol synthesis.
Acetoacetate: This is one of the three ketone bodies produced during ketogenesis, formed from the cleavage of HMG CoA. Acetoacetate is a metabolic fuel that can be transported through the blood and used by peripheral tissues for energy.
β-Hydroxybutyrate: The most abundant and stable ketone body, formed by the reduction of acetoacetate. β-Hydroxybutyrate is the primary ketone body found in the blood and is a significant fuel source for the brain, heart, and muscle, particularly during prolonged fasting or ketogenic diets.
The Role of Ketone Bodies in Energy Metabolism
In times of abundant glucose, the brain primarily relies on this simple sugar for its energy needs. However, during prolonged fasting, starvation, or when following a very low-carbohydrate, high-fat ketogenic diet, glucose supplies become limited. In such physiological states, the body must find alternative fuel sources to sustain the high energy demands of organs like the brain, which cannot efficiently utilize fatty acids directly. This is where ketogenesis, the metabolic pathway for synthesizing ketone bodies, becomes critically important.
Ketogenesis occurs exclusively in the mitochondria of liver cells. When the rate of fatty acid oxidation in the liver is high (due to low insulin levels and high glucagon levels), it generates an abundance of acetyl CoA. This excess acetyl CoA, exceeding the liver’s capacity to process it through the Krebs cycle, is then diverted into the ketogenesis pathway. The liver itself cannot utilize the ketone bodies it produces; instead, it releases them into the bloodstream for use by other tissues.
The three main ketone bodies are acetoacetate, β-hydroxybutyrate, and acetone. Acetoacetate and β-hydroxybutyrate are the primary energy-yielding ketone bodies, readily transported to extrahepatic tissues (tissues outside the liver) such as the brain, heart, and skeletal muscle. Once in these tissues, ketone bodies can be converted back into acetyl CoA and fed into the Krebs cycle, thereby providing a vital source of ATP. Acetone, a volatile compound, is produced in smaller amounts and is typically exhaled.
- Ketogenesis produces ketone bodies from acetyl CoA.
- It occurs in the mitochondria of liver cells.
- Ketone bodies serve as alternative fuel, especially for the brain.
- It is activated during fasting or low carbohydrate intake.
The Biochemical Pathway of Ketogenesis
The synthesis of ketone bodies begins with the condensation of two molecules of acetyl CoA to form acetoacetyl CoA, a reaction catalyzed by thiolase. This initial step is essentially the reverse of the final step of beta-oxidation. Subsequently, a third molecule of acetyl CoA condenses with acetoacetyl CoA to form β-hydroxy-β-methylglutaryl CoA (HMG CoA). This crucial step is catalyzed by HMG-CoA synthase, an enzyme located in the mitochondrial matrix.
HMG CoA is a key intermediate because its fate dictates whether the carbon atoms proceed towards ketone body synthesis or cholesterol synthesis (which occurs in the cytosol). In the mitochondrial ketogenesis pathway, HMG CoA is cleaved by HMG-CoA lyase, releasing one molecule of acetyl CoA and forming acetoacetate. Acetoacetate is the first true ketone body produced and can either be released directly into the bloodstream or undergo further transformation.
Acetoacetate can then be reduced to β-hydroxybutyrate by the enzyme β-hydroxybutyrate dehydrogenase, a reversible reaction that utilizes NADH. This interconversion means that acetoacetate and β-hydroxybutyrate exist in equilibrium, with the ratio depending on the cellular redox state (NADH/NAD+ ratio). β-Hydroxybutyrate is the most stable and abundant ketone body in the blood. Finally, acetoacetate can also spontaneously decarboxylate to form acetone, a non-metabolizable ketone body that is excreted through respiration, giving a characteristic fruity smell to the breath during severe ketosis.
Clinical Significance and Ketosis
The physiological state of elevated ketone bodies in the blood is known as ketosis. Mild nutritional ketosis, often achieved through ketogenic diets, is generally considered safe and can offer potential health benefits such as weight loss and improved metabolic markers. However, an uncontrolled and excessive production of ketone bodies can lead to a dangerous condition called ketoacidosis, particularly in individuals with Type 1 diabetes mellitus.
In Type 1 diabetes, a severe insulin deficiency leads to unchecked fatty acid breakdown and massive production of acetyl CoA. Without sufficient insulin, glucose cannot enter cells, and the body incorrectly perceives a state of starvation, despite high blood glucose levels. This triggers profound ketogenesis, leading to a build-up of acidic ketone bodies in the blood (acetoacetate and β-hydroxybutyrate are acids). The resulting metabolic acidosis can be life-threatening, causing symptoms like nausea, vomiting, abdominal pain, Kussmaul respiration, and eventually coma. Diabetic ketoacidosis (DKA) requires immediate medical attention, typically involving insulin administration and fluid replacement.
Understanding ketogenesis is also crucial in the context of therapeutic ketogenic diets, which are used to manage certain neurological disorders, such as refractory epilepsy. By shifting the brain’s primary fuel source from glucose to ketone bodies, these diets can have anticonvulsant effects. Researchers are also exploring the potential role of ketogenic diets in other conditions, including Alzheimer’s disease and some cancers, highlighting the multifaceted importance of this metabolic pathway in human health and disease.
Conclusion
Ketogenesis is a vital adaptive metabolic response that allows the body to generate alternative fuel sources—ketone bodies—during periods of glucose scarcity. Occurring exclusively in the liver, this pathway converts excess acetyl CoA into acetoacetate and β-hydroxybutyrate, which can then be utilized by extrahepatic tissues, most notably the brain. While controlled ketosis can offer therapeutic benefits, unregulated ketogenesis, as seen in diabetic ketoacidosis, underscores the critical importance of maintaining metabolic balance and the profound implications of dysregulation within this essential pathway.

