Hemoglobin is a critical protein within red blood cells, designed to transport oxygen from the lungs to tissues and facilitate carbon dioxide removal, playing an indispensable role in respiration. This diagram provides a detailed view of the hemoglobin molecule’s heme group, highlighting its chemical composition and the iron center that enables oxygen binding. Exploring this structure offers valuable insights into its function and the broader context of oxygen delivery in the body.
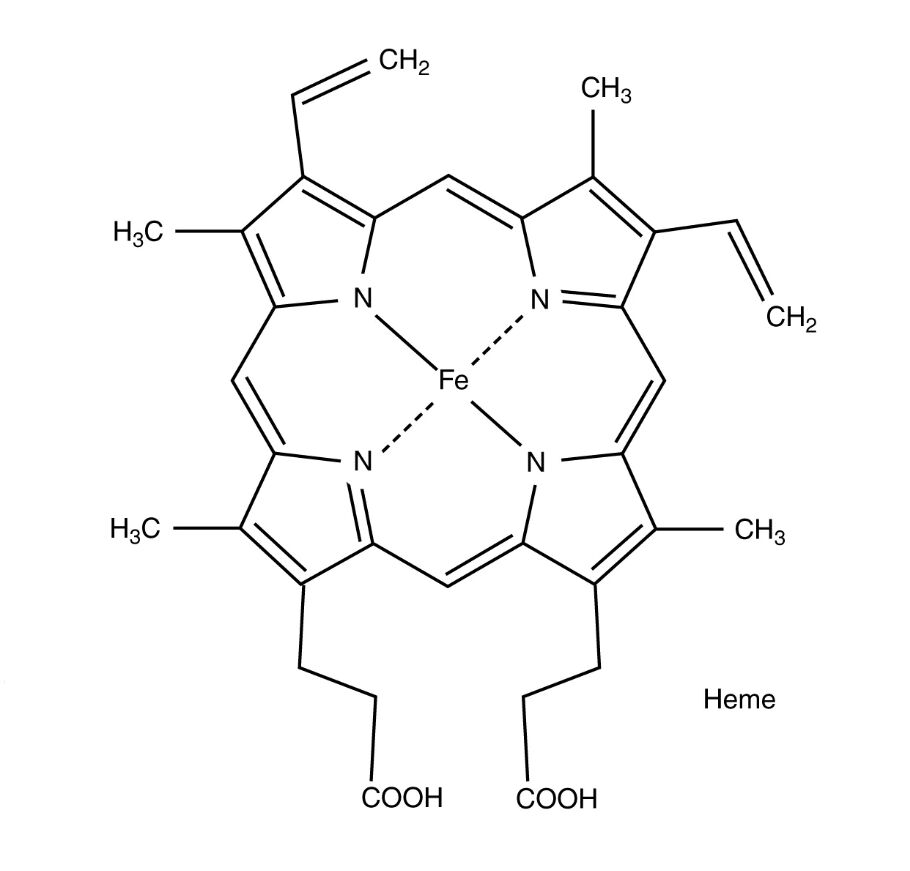
Key Components of the Hemoglobin Molecule Structure
The diagram focuses on the essential elements that define the heme group’s role.
Heme:
Heme is the iron-containing pigment that forms the core of hemoglobin’s oxygen-binding capability, embedded within each globin chain of the protein. Its complex structure, featuring a porphyrin ring, allows it to reversibly bind oxygen, making it essential for effective gas exchange.
Fe:
The iron atom at the center of the heme group, in its ferrous (Fe²⁺) state, serves as the binding site for oxygen molecules. This iron’s ability to switch oxidation states facilitates the dynamic attachment and release of oxygen based on tissue needs.
CH₂:
These methylene groups are part of the side chains extending from the porphyrin ring, contributing to the heme’s stability and flexibility. They help anchor the heme within the globin protein, ensuring proper orientation for oxygen binding.
CH₃:
The methyl groups attached to the porphyrin ring enhance the heme’s hydrophobic environment, stabilizing the iron center. They play a role in maintaining the structural integrity of the heme group during oxygen transport.
COOH:
These carboxyl groups are located at the periphery of the heme molecule, providing polarity and aiding in its solubility within the aqueous environment of blood. They also contribute to the heme’s interaction with the globin chains, securing its position.
H₃C:
The methyl groups denoted as H₃C are additional side chains that reinforce the heme’s chemical stability, supporting the iron’s ability to bind oxygen. They are integral to the porphyrin ring’s overall structure and function.
The Anatomical and Physiological Role of Hemoglobin
The heme group is the functional heart of hemoglobin, enabling its primary role as an oxygen carrier within red blood cells. The iron atom (Fe) at its center binds oxygen in the lungs, where high partial pressure favors attachment, and releases it in tissues with lower oxygen levels, a process influenced by the Bohr effect and carbon dioxide concentration. This reversible binding is made possible by the porphyrin ring, a tetrapyrrole structure stabilized by CH₂, CH₃, and COOH groups, which create a hydrophobic pocket within the globin protein.
Hemoglobin’s ability to transport carbon dioxide, albeit to a lesser extent (about 10% as carbaminohemoglobin), aids in maintaining acid-base balance alongside the chloride shift. The production of hemoglobin is regulated by erythropoietin, a hormone from the kidneys, which responds to hypoxia, while thyroid hormones like T3 and T4 indirectly influence metabolic oxygen demand. The heme’s structure ensures that each hemoglobin molecule, containing four heme groups, can carry up to four oxygen molecules, optimizing oxygen delivery.
- Oxygen Binding: The ferrous iron shifts to a high-spin state upon oxygen binding; this change enhances affinity for additional oxygen molecules.
- Structural Support: Methyl and methylene groups prevent heme degradation; carboxyl groups interact with globin histidine residues.
- Physiological Regulation: Erythropoietin levels rise with altitude; hemoglobin synthesis requires iron and vitamin B12.
Physical Characteristics and Clinical Relevance
The heme group’s physical structure, as depicted, is a flat, circular porphyrin ring with a central iron atom, designed for efficient oxygen coordination. The presence of COOH groups adds a degree of polarity, while CH₃ and CH₂ groups contribute to its hydrophobic nature, ensuring stability within the red blood cell’s aqueous environment. This structure allows a single hemoglobin molecule to transport over 1 billion oxygen molecules across its lifespan.
Clinically, the heme group’s integrity is critical for diagnosing blood disorders. Abnormalities in iron metabolism, such as iron-deficiency anemia, reduce heme synthesis, lowering oxygen-carrying capacity. Conditions like porphyrias, caused by heme biosynthesis defects, lead to toxic precursor accumulation. Laboratory tests, including serum iron levels and hemoglobin electrophoresis, assess heme function, guiding treatments like iron supplementation or chelation therapy for overload.
- Diagnostic Approaches: Mean corpuscular hemoglobin (MCH) reflects heme content per cell; ferritin levels indicate iron stores.
- Therapeutic Options: Intravenous iron corrects severe deficiency; phlebotomy manages hemochromatosis-related heme excess.
Conclusion
The hemoglobin molecule structure diagram, with its focus on the heme group, reveals the elegant chemistry that underpins oxygen transport and carbon dioxide management in the body. This molecular arrangement not only supports vital respiratory functions but also serves as a key indicator of hematological health. By understanding the roles of its components, one can better appreciate the intricate balance required to maintain oxygenation and the importance of monitoring heme-related conditions for optimal well-being.

