Postsynaptic potential summation is a critical process in how neurons integrate signals to determine their response, shaping the overall change in membrane potential. This article delves into the mechanisms depicted in the provided image, where excitatory and inhibitory signals converge to influence neuronal activity. By understanding this process, one can gain deeper insight into the complex communication network within the nervous system.
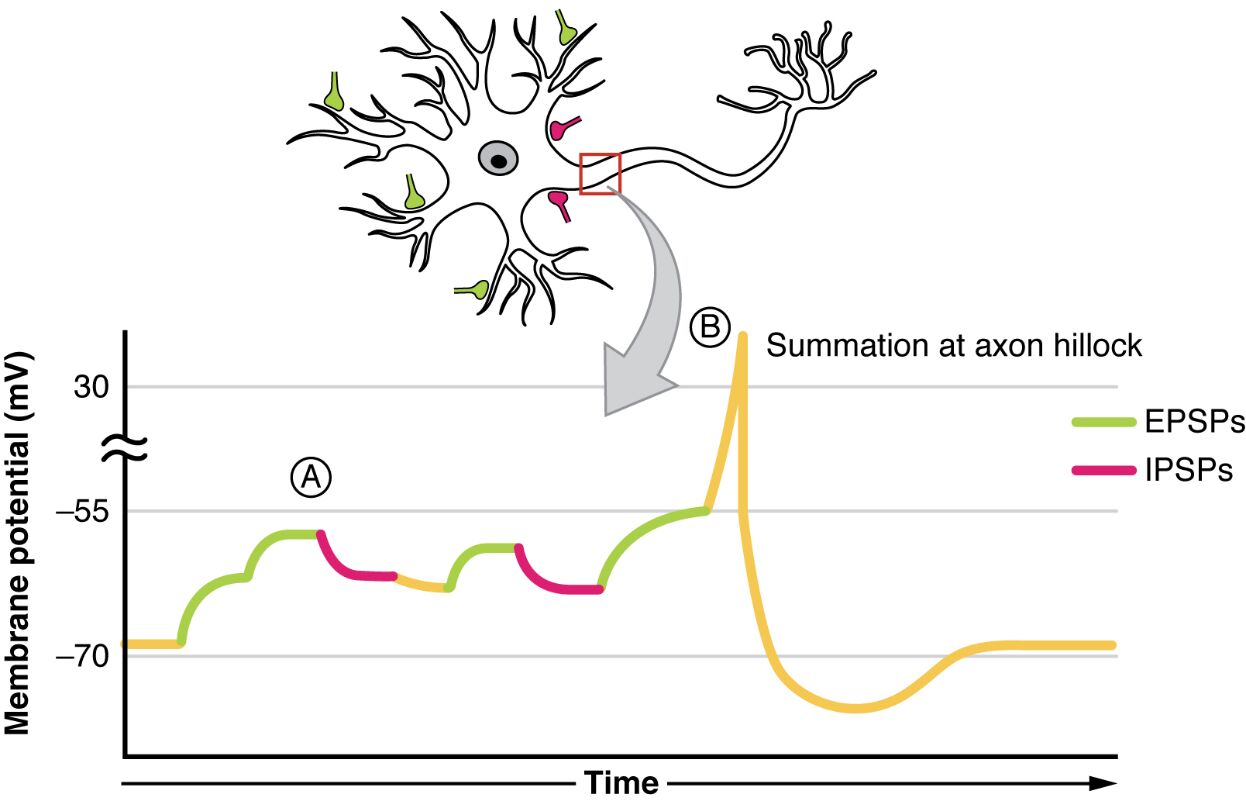
A: This label marks the point where multiple excitatory postsynaptic potentials (EPSPs) combine, leading to a significant depolarization of the membrane. The summation at this stage can bring the membrane potential closer to the threshold needed for an action potential.
B: This indicates the axon hillock where summation of both excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) occurs, resulting in a varied membrane potential outcome. The balance between these opposing forces determines whether an action potential is triggered.
EPSPs: These green traces represent excitatory postsynaptic potentials, which result from the influx of sodium or calcium ions, causing depolarization. They play a key role in increasing the likelihood of an action potential by moving the membrane potential toward the threshold.
IPSPs: Shown as red traces, inhibitory postsynaptic potentials occur due to the influx of chloride ions or efflux of potassium ions, leading to hyperpolarization. They decrease the likelihood of an action potential by moving the membrane potential away from the threshold.
Summation at axon hillock: This process involves the integration of EPSPs and IPSPs at the axon hillock, a critical decision point for neuronal firing. The net effect depends on the timing and strength of these potentials, influencing whether the neuron will propagate a signal.
The Mechanism of Postsynaptic Potential Summation
Summation is the cornerstone of neuronal integration, allowing cells to process multiple inputs effectively. This process ensures that the neuron responds appropriately to the combined effect of excitatory and inhibitory signals.
- Postsynaptic potential summation occurs when EPSPs and IPSPs overlap in time or space, altering the membrane potential.
- Temporal summation happens when repeated stimuli from the same synapse add up over time.
- Spatial summation involves inputs from different synapses converging on the same neuron.
- The axon hillock serves as the integration site due to its high density of voltage-gated sodium channels.
- This mechanism allows neurons to filter and prioritize signals based on their combined strength.
Anatomical and Physiological Insights
The structure of a neuron is intricately designed to support summation, with specific regions playing distinct roles. Understanding the anatomy and physiology behind this process enhances comprehension of neural signaling.
- The dendrites receive initial synaptic inputs, where EPSPs and IPSPs are generated.
- The cell body integrates these potentials, transmitting them toward the axon hillock.
- The axon hillock, rich in ion channels, acts as the trigger zone for action potentials.
- Voltage-gated sodium channels open when the threshold of approximately -55 mV is reached.
- This anatomical arrangement ensures efficient signal processing and transmission.
Clinical Relevance and Neural Communication
While summation itself is not a disease, its disruption can contribute to neurological disorders. Proper integration of postsynaptic potentials is vital for healthy brain function, influencing everything from reflexes to cognition.
- Imbalances in EPSPs and IPSPs can lead to hyperexcitability, seen in conditions like epilepsy.
- Deficiencies in inhibitory control may result in uncontrolled neuronal firing.
- The process supports sensory processing, allowing the brain to interpret complex stimuli.
- Therapeutic interventions often target ion channels to modulate summation.
- Understanding this can aid in developing treatments for neural dysregulation.
Postsynaptic potential summation is a fascinating aspect of neuronal physiology, bridging the gap between individual synaptic events and coordinated neural responses. The image effectively illustrates how EPSPs and IPSPs interact at the axon hillock, offering a clear visual of this dynamic process. By mastering these concepts, one can better appreciate the precision of neural communication and its role in maintaining bodily functions, paving the way for further exploration into neural network behavior.

