The development of the human heart reaches a sophisticated stage by day 35, where the embryonic cardiovascular system begins to resemble its mature form with structures like the right atrium, left atrium, ventricle, truncus arteriosus, and aortic arch arteries. This image captures the heart’s ongoing transformation, illustrating the segmentation and early vascular connections that are vital for sustaining the embryo as it grows into a more complex organism.
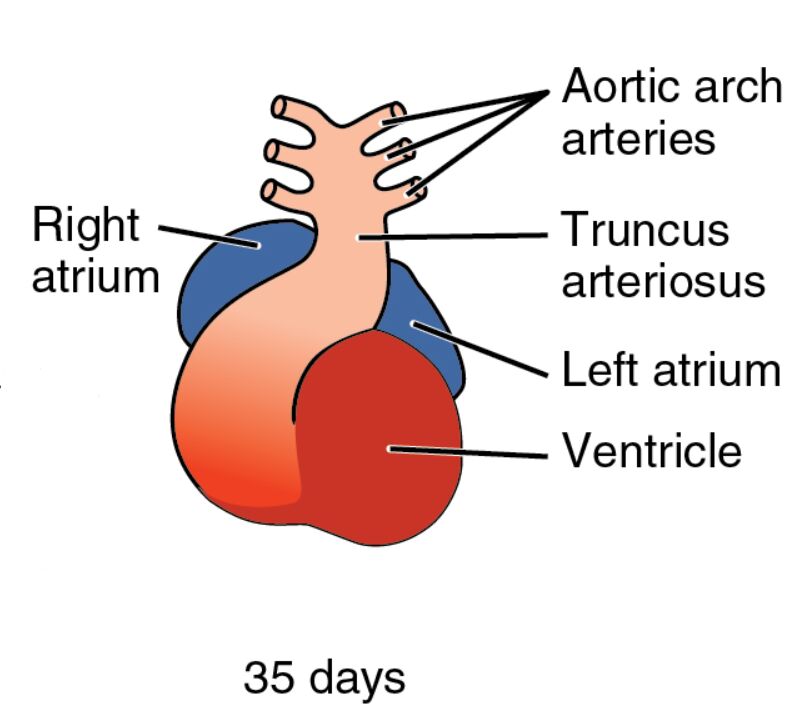
Right atrium Right atrium serves as the receiving chamber for deoxygenated blood returning from the body, positioned on the right side of the developing heart. This structure will eventually incorporate the sinus venosus and contribute to the right atrial chamber in the mature heart.
Left atrium Left atrium receives oxygenated blood from the primitive pulmonary veins, located on the left side of the heart tube. It will expand and develop pectinate muscles, playing a key role in future left atrial function.
Ventricle Ventricle acts as the primary pumping chamber, driving blood out through the truncus arteriosus, and is centrally located in the heart tube. This region will divide into the left and right ventricles, each tailored for systemic or pulmonary circulation.
Truncus arteriosus Truncus arteriosus forms the common outflow tract, channeling blood from the ventricle to the aortic arch arteries, and is situated at the heart’s cranial end. This structure will undergo septation to form the aorta and pulmonary artery, a critical step in establishing dual circulation.
Aortic arch arteries Aortic arch arteries are the early vascular structures branching from the truncus arteriosus, supplying blood to the developing pharyngeal arches. These arteries will remodel into the major arteries of the head, neck, and thorax, including the carotid and subclavian arteries.
35 days 35 days marks the gestational age, aligning with Carnegie stage 14-15, when the heart exhibits clear chamber formation and vascular connections. The embryo, now about 6-9 mm long, undergoes rapid organogenesis, with the heart playing a central role in its circulatory support.
The Role of Chamber Formation in Heart Development
This stage highlights the heart’s progression toward a four-chambered structure. The differentiation of atria and ventricles marks a significant milestone in embryonic circulation.
- Atrial Development: The right and left atria begin to separate, guided by the septum primum and septum secundum. This separation ensures proper blood flow partitioning between systemic and pulmonary circuits.
- Ventricular Growth: The ventricle thickens its myocardial walls, enhancing its pumping capacity. Its division into two chambers is driven by the interventricular septum.
- Outflow Tract Evolution: The truncus arteriosus starts its septation process, influenced by neural crest cell migration. This division is essential for separating pulmonary and systemic blood flow.
- Vascular Integration: The aortic arch arteries connect to the developing arterial system, supporting the embryo’s head and neck. These connections will refine into the adult arterial pattern.
Anatomical Insights into the 35-Day Embryo
The image reveals the heart’s S-shaped loop, with distinct color gradients indicating blood flow through its chambers. This visual representation underscores the complexity of early cardiac anatomy.
The embryo’s size and folding continue to protect and position the heart within the thoracic cavity. The emerging chambers and arteries reflect the heart’s adaptation to increasing circulatory demands.
- Right Atrium Anatomy: This chamber receives blood via the superior and inferior vena cava precursors. Its development includes the incorporation of the right horn of the sinus venosus.
- Left Atrium Structure: The left atrium expands as pulmonary veins connect, marking the onset of lung circulation. Its growth is supported by surrounding mesenchymal tissue.
- Ventricle Characteristics: The ventricle’s thick walls indicate its role as the primary pump, with trabeculae forming to enhance contraction. Its division will create the left and right ventricles.
- Truncus Arteriosus Features: This outflow tract contains a spiral septum forming under neural crest influence. Its division will establish the aorta and pulmonary trunk.
- Aortic Arch Arteries Details: These arteries arise from the pharyngeal arches, with arches 3, 4, and 6 contributing to major vessels. Their remodeling is guided by hemodynamic forces.
Developmental Milestones at 35 Days
This phase signifies rapid advancements in heart development, with chamber formation driving circulatory efficiency. The integration of vascular and cardiac structures highlights the embryo’s progress.
The neural tube is fully closed, and limb buds begin to form, paralleling cardiac maturation. These milestones reflect the synchronized growth of multiple systems.
- Chamber Separation: The atrial and ventricular septa begin to develop, guided by NKX2-5 and GATA4 genes. This separation prevents abnormal shunting of blood.
- Looping Refinement: The S-shape loop adjusts to accommodate chamber growth, influenced by Pitx2 signaling. This alignment ensures proper heart orientation.
- Vascular Maturation: The aortic arch arteries undergo selective regression and persistence. Arches 1 and 2 regress, while others form key arteries.
- Hematopoietic Support: Blood islands in the yolk sac continue producing primitive erythrocytes. This supports the heart’s circulatory demands until the liver takes over.
Physiological Functions of the Developing Heart
The 35-day heart begins to perform complex physiological roles, supporting the embryo’s growing needs. Its segmented chambers enhance blood distribution and oxygenation.
The heart’s contractions strengthen, driven by a maturing conduction system. This efficiency prepares the embryo for the transition to fetal circulation.
- Pumping Mechanism: The ventricle’s thickened walls enable stronger contractions, propelling blood through the truncus arteriosus. This flow supports embryonic growth.
- Oxygenation Process: Oxygenated blood from the placenta enters the left atrium, while deoxygenated blood returns to the right atrium. This dual flow anticipates lung function post-birth.
- Pressure Regulation: The developing chambers create pressure gradients, aiding vascular remodeling. These gradients influence arterial development.
- Future Adaptations: Shunts like the ductus arteriosus begin to form, bypassing the lungs. This adaptation optimizes circulation in the intrauterine environment.
Clinical and Research Implications
The 35-day embryo provides critical insights for medical practice and research. The heart’s chamber formation is a key focus for studying congenital defects.
Advanced imaging and stem cell technologies enhance our understanding of this stage. These tools offer potential for early diagnosis and innovative treatments.
- Congenital Anomalies: Incomplete septation can lead to atrial septal defects. Fetal echocardiography detects these issues, guiding prenatal care.
- Regenerative Approaches: Stem cells can differentiate into atrial and ventricular cardiomyocytes. This holds promise for repairing congenital heart conditions.
- Animal Models: Chick and mouse embryos mirror human chamber development. These models validate research findings.
- Therapeutic Advances: Targeting VEGF pathways may enhance vascular growth in defects. Gene editing explores correcting cardiac malformations.
In conclusion, this image of the 35-day embryo showcases the heart’s evolution into distinct chambers and vascular connections, a vital step in cardiovascular development. This early structuring not only supports embryonic life but also offers a foundation for understanding and addressing heart-related health challenges.

