The cardiac sarcomere is the fundamental contractile unit of heart muscle cells, responsible for the rhythmic beating that sustains life. This intricate assembly of proteins facilitates the crucial sliding filament mechanism, allowing the heart to pump blood effectively. Understanding its various components provides essential insight into myocardial function and the underpinnings of cardiovascular health.
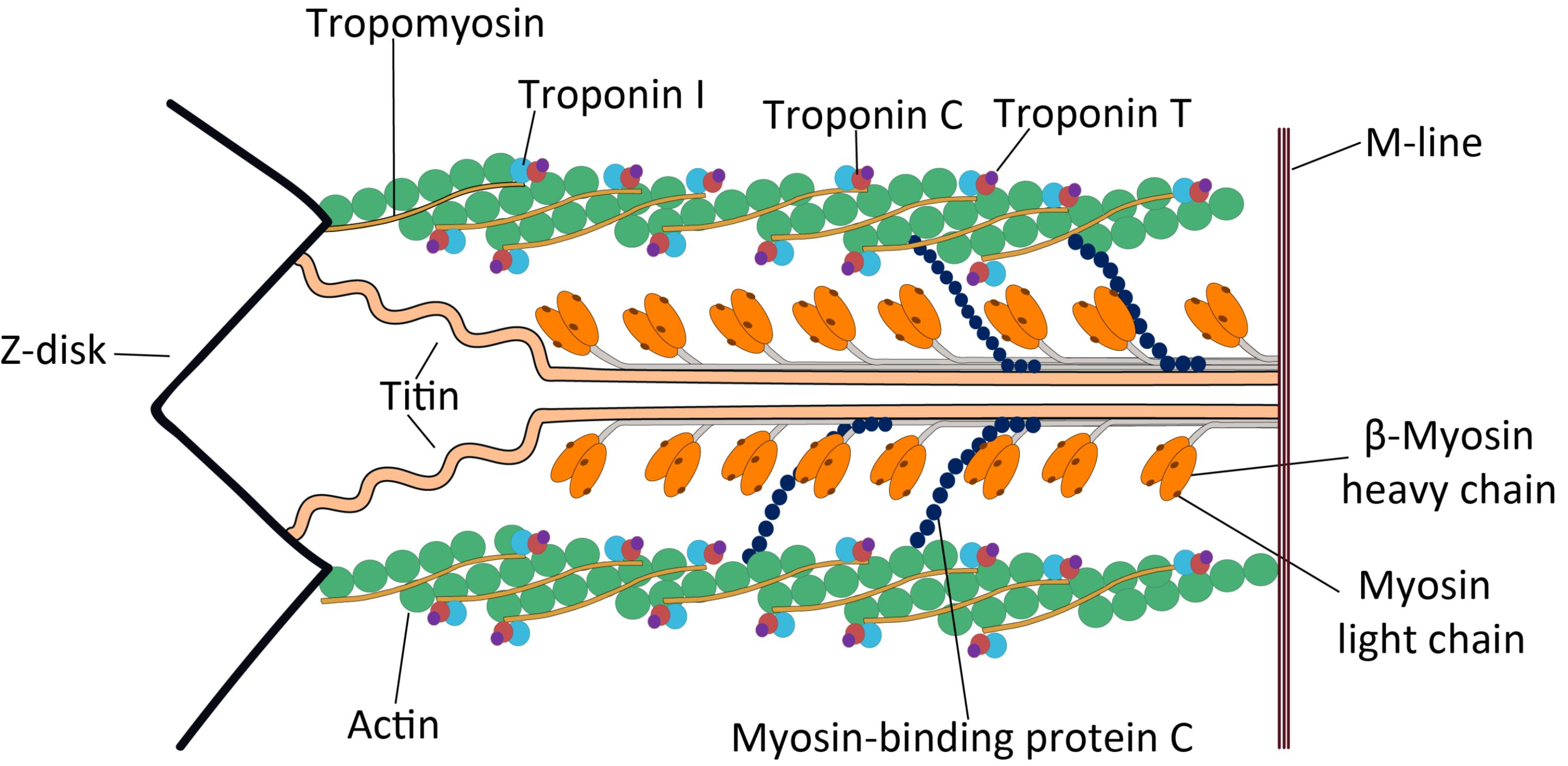
Tropomyosin: Tropomyosin is a long, fibrous protein that extends along the actin filaments in the sarcomere. Its primary role is to regulate muscle contraction by covering the myosin-binding sites on actin in the absence of calcium, thus preventing unwanted muscle activation.
Troponin I: Troponin I (inhibitory) is one of the three subunits of the troponin complex, binding to actin to hold the tropomyosin in its inhibitory position. This subunit’s function is to prevent myosin from binding to actin when the muscle is at rest.
Troponin C: Troponin C (calcium-binding) is the subunit of the troponin complex that binds calcium ions. The binding of calcium to Troponin C initiates a conformational change that ultimately leads to the uncovering of myosin-binding sites on actin, allowing contraction to proceed.
Troponin T: Troponin T (tropomyosin-binding) is the subunit of theponin complex that attaches the entire troponin complex to the tropomyosin molecule. This attachment ensures that the troponin complex is properly positioned on the actin filament to regulate contraction.
M-line: The M-line is a dense line in the center of the sarcomere, serving as the anchoring point for the thick myosin filaments. It plays a crucial role in maintaining the structural integrity and organization of the myosin filaments within the sarcomere.
Z-disk: The Z-disk, or Z-line, defines the boundaries of a single sarcomere, acting as the anchoring point for the thin actin filaments. During muscle contraction, the Z-disks move closer together, shortening the sarcomere.
Titin: Titin is an exceptionally large, elastic protein that extends from the Z-disk to the M-line, coiling around the thick myosin filaments. It acts as a molecular spring, contributing to the passive elasticity of muscle and preventing overstretching of the sarcomere.
β-Myosin heavy chain: The β-Myosin heavy chain forms the core structural component of the thick myosin filament in cardiac muscle. It possesses the ATPase activity and actin-binding sites essential for generating force during muscle contraction.
Myosin light chain: Myosin light chains are small proteins associated with the globular heads of the myosin heavy chains. They play a regulatory role in muscle contraction, modulating the ATPase activity of the myosin head and influencing its interaction with actin.
Actin: Actin is the primary component of the thin filaments in the sarcomere, forming a helical structure to which myosin heads bind during muscle contraction. It provides the track along which the myosin heads “walk” to shorten the muscle.
Myosin-binding protein C: Myosin-binding protein C (MyBP-C) is a structural protein associated with the thick myosin filaments, located in the C-zone of the sarcomere. It plays a significant role in regulating myocardial contraction and relaxation, and its phosphorylation status can modulate cross-bridge cycling.
The cardiac sarcomere stands as the fundamental unit of contraction within every heart muscle cell, acting as the microscopic engine that drives the circulatory system. This highly organized assembly of proteins is meticulously arranged to facilitate the sliding filament mechanism, enabling the heart to pump blood with remarkable efficiency and rhythmic precision. A deep understanding of the sarcomere’s intricate structure and the function of its various components is not merely academic; it is crucial for comprehending the physiological basis of heart function and the pathophysiology of numerous cardiac diseases, ranging from heart failure to cardiomyopathies.
Within the sarcomere, thin filaments, primarily composed of actin, and thick filaments, primarily composed of myosin, interact in a highly coordinated fashion. Regulatory proteins such as troponin and tropomyosin govern this interaction, ensuring that contraction only occurs when appropriate signals are received, primarily through calcium influx. Structural proteins like titin and myosin-binding protein C contribute to the sarcomere’s elasticity, stability, and overall mechanical performance. This precise arrangement and dynamic interplay allow the heart muscle to generate the powerful forces required for continuous blood circulation throughout a lifetime.
Dysfunction in any of these intricate components can lead to profound effects on cardiac contractility and overall heart health. Genetic mutations affecting sarcomeric proteins are a common cause of inherited cardiomyopathies, highlighting the critical role these structures play in maintaining cardiac function. Exploring the individual proteins within the sarcomere reveals a symphony of molecular interactions, each vital for the heart’s ability to contract and relax effectively.
- The sarcomere is bounded by Z-disks, where thin actin filaments are anchored.
- Thick myosin filaments are centrally anchored at the M-line.
- The interaction between actin and myosin, regulated by troponin and tropomyosin, drives contraction.
- Titin provides elasticity, while myosin-binding protein C modulates contraction.
The Actin-Myosin Dance: The Core of Contraction
At the heart of cardiac muscle contraction is the interaction between actin and myosin filaments, often described as the sliding filament theory. Actin, forming the thin filaments, is a double-helical polymer that provides binding sites for the myosin heads. Myosin, forming the thick filaments, consists of heavy chains with globular heads that possess ATPase activity and can bind to actin. During contraction, the myosin heads attach to actin, pivot, and detach in a cyclical manner, pulling the actin filaments towards the center of the sarcomere, thus shortening the muscle. This process is highly energy-dependent, relying on ATP hydrolysis to fuel the conformational changes in the myosin head.
Regulatory Proteins: Troponin and Tropomyosin
The precise control of the actin-myosin interaction is orchestrated by the regulatory proteins tropomyosin and the troponin complex. In a resting state, tropomyosin strands lie along the actin filaments, physically blocking the myosin-binding sites. The troponin complex, consisting of three subunits—Troponin I, Troponin T, and Troponin C—is strategically positioned on the tropomyosin molecule. When an electrical impulse triggers the release of calcium ions into the cardiac myocyte, calcium binds to Troponin C. This binding induces a conformational change in the troponin complex, which in turn shifts the tropomyosin molecule away from the myosin-binding sites on actin. With these sites exposed, myosin heads can now bind to actin, initiating the cross-bridge cycle and muscle contraction.
Structural and Modulatory Proteins: Titin and Myosin-binding Protein C
Beyond the primary contractile and regulatory proteins, the cardiac sarcomere incorporates essential structural and modulatory proteins that ensure its integrity, elasticity, and fine-tuning of contraction. Titin, a massive elastic protein, acts as a molecular spring, connecting the Z-disk to the M-line. It provides passive stiffness to the muscle, preventing overstretching and helping the sarcomere return to its resting length after contraction. Myosin-binding protein C (MyBP-C) is another crucial protein located in the thick filaments. It helps organize the myosin filaments and plays a significant role in modulating the kinetics of myosin cross-bridge cycling and force generation. Phosphorylation of MyBP-C by protein kinases like PKA can alter its function, thus influencing the speed and force of cardiac contraction.
Clinical Relevance: Sarcomeric Cardiomyopathies
Dysfunction or mutations in the genes encoding sarcomeric proteins are a primary cause of inherited cardiomyopathies, a group of diseases affecting the heart muscle. Hypertrophic cardiomyopathy (HCM), for instance, is frequently caused by mutations in genes for β-Myosin heavy chain, myosin light chains, or myosin-binding protein C. These mutations often lead to an abnormal thickening of the heart muscle, impaired relaxation, and an increased risk of sudden cardiac death. Dilated cardiomyopathy (DCM) can also arise from sarcomeric protein mutations, leading to ventricular dilation and systolic dysfunction. Understanding the specific protein affected helps in genetic counseling, diagnosis, and the development of targeted therapies for these debilitating heart conditions.
The cardiac sarcomere, with its meticulously arranged array of proteins, represents a marvel of biological engineering. Each component, from the contractile actin and myosin to the regulatory troponin and tropomyosin, and the structural titans like titin and MyBP-C, plays an indispensable role in the heart’s ability to contract and pump blood efficiently. Continued research into the precise functions and interactions of these proteins is vital for advancing our understanding of cardiovascular health and for developing novel therapeutic strategies for heart diseases that stem from sarcomeric dysfunction.

