Discover how scientists are pushing the boundaries of personalized medicine with a groundbreaking lung-on-a-chip model. This innovative device, crafted from a single donor’s cells, mimics real breathing and early infection stages, offering fresh hope for tackling tough respiratory diseases like tuberculosis. It’s not just lab stuff—it’s a step toward treatments tailored to your unique biology.
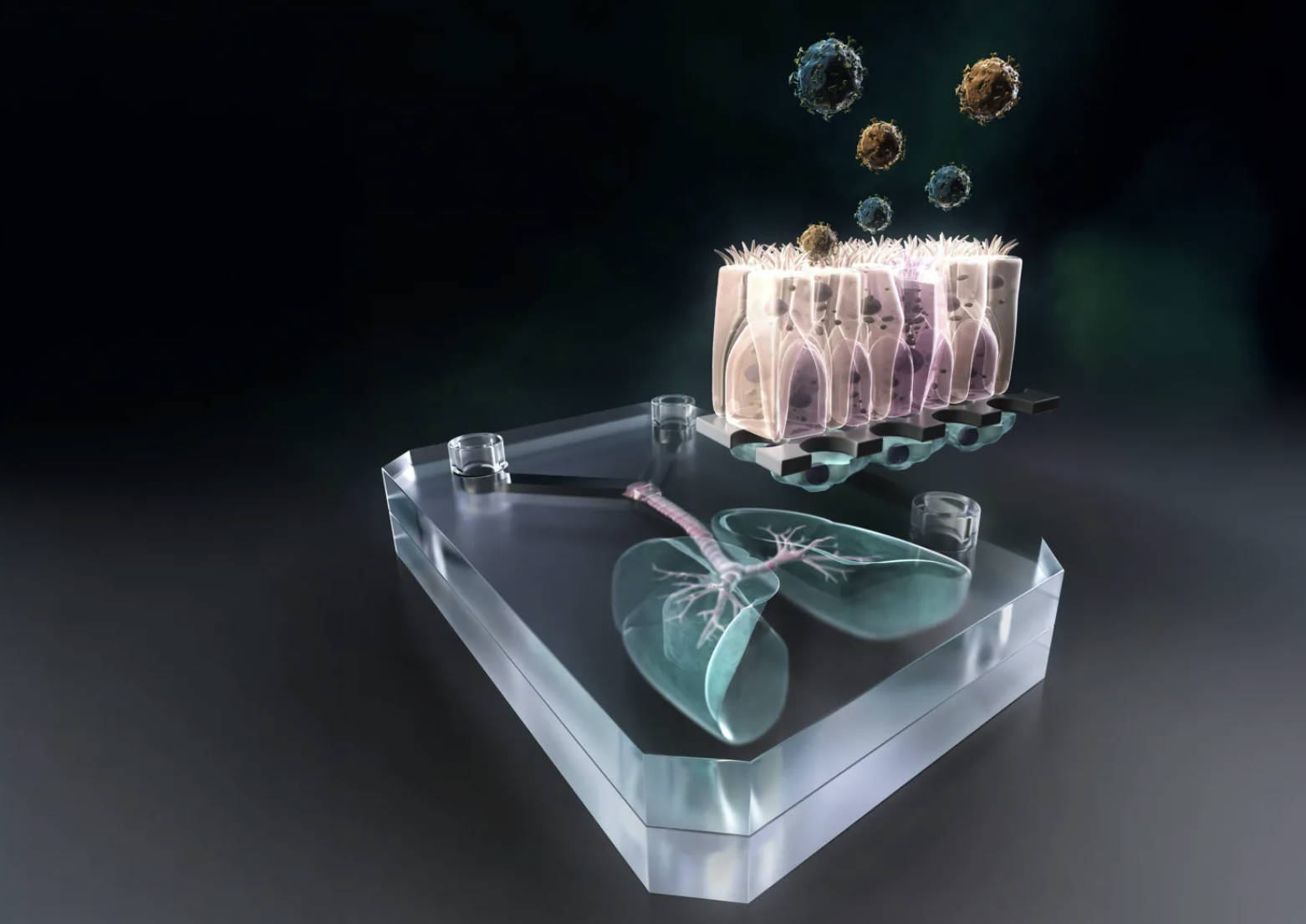
- What Makes It Special? Unlike older models mixing cells from different sources, this one uses genetically identical stem cells from one person, creating a mini-lung that acts just like the real thing in that individual.
- How It Works: Researchers grow lung and blood vessel cells on a super-thin membrane, then add a stretching mechanism to simulate breathing, helping cells develop properly and respond to threats.
- Testing Infections: They introduced TB bacteria and watched immune cells cluster up, form dead zones, and eventually break down barriers—mirroring what happens in early, hard-to-spot stages of the disease.
- Big Picture Impact: This could speed up drug testing, reveal why some people fight infections better than others, and pave the way for custom therapies without risking patients.
- Who’s Behind It? A collaboration between the UK’s Francis Crick Institute and Switzerland’s AlveoliX, highlighting global teamwork in biotech.
First Genetically Matched Human Lung-on-a-Chip Emerges, Offering New Tools for Personalized Respiratory Disease Treatment
According to a report in Science Advances published on January 1, 2026, a collaborative team from the Francis Crick Institute in the UK and the Swiss biotechnology company AlveoliX has developed the world’s first genetically matched human “lung-on-a-chip” model. This innovative chip utilizes stem cells from a single donor to simulate individual alveolar breathing movements and infection responses, providing powerful new tools for personalized treatment of respiratory diseases such as tuberculosis.
Alveoli serve as the lungs’ critical sites for gas exchange while also acting as a vital barrier against inhaled viruses and bacteria. For years, scientists have sought to recreate the dynamic interactions between human cells and pathogens in a lab setting. One approach has been the creation of lung chips—miniature devices that integrate tiny channels and compartments on plastic substrates to form scaled-down human lung tissue units. However, traditional lung chips often relied on a blend of patient-derived and commercially available cells, which made it challenging to accurately replicate the lung function and disease progression of any one individual.
Building on prior lab protocols, the team differentiated human induced pluripotent stem cells into type I and type II alveolar epithelial cells, as well as vascular endothelial cells. These were then cultured on either side of an ultra-thin membrane produced by AlveoliX, effectively reconstructing the alveolar barrier structure.
To more closely mimic the authentic human lung environment, AlveoliX engineered a specialized device that applies rhythmic three-dimensional stretching forces to the chip. This simulates natural breathing motions and encourages the formation of microvilli on the alveolar epithelial cells.
The researchers then incorporated macrophages—differentiated from the same donor’s stem cells—into the chip and introduced Mycobacterium tuberculosis to model the early phases of infection. Observations revealed the formation of large macrophage clusters within the infected chip, featuring a central “necrotic core” of dead macrophages encircled by surviving cells. By day five post-infection, the epithelial and endothelial barriers had collapsed, impairing alveolar function and vividly capturing the pathological hallmarks of early-stage infection.
Tuberculosis typically takes months from initial infection to symptom onset, making early changes notoriously difficult to study. This new chip successfully recreates the lung’s initial responses to infection, delivering a comprehensive view that enhances our understanding of disease progression.
lung-on-a-chip, genetically matched, human stem cells, alveolar model, tuberculosis infection, personalized medicine, respiratory diseases, breathing simulation, macrophage clusters, necrotic core, alveolar barrier, induced pluripotent stem cells, Science Advances, Francis Crick Institute, AlveoliX

