The immune system’s strength lies in the coordinated efforts of various cell types, with B cells and T cells playing pivotal roles in adaptive immunity. This image captures the critical interaction between these cells during a response to a T cell-dependent antigen, highlighting the dual signals required for full B cell activation. Exploring this process reveals the intricate cellular communication that underpins effective pathogen defense and long-term immune memory.
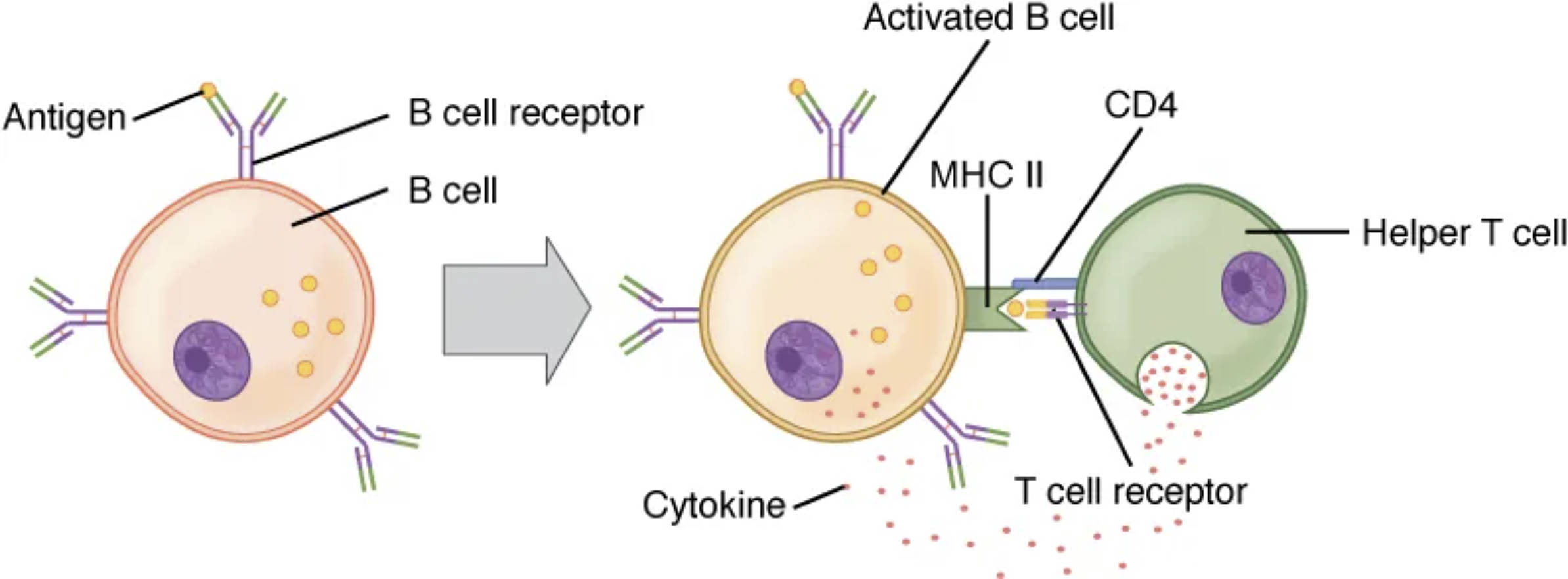 Key Labels in the B and T Cell Interaction Diagram
Key Labels in the B and T Cell Interaction Diagram
This section provides a detailed look at each labeled component, shedding light on their roles in immune activation.
Antigen: This foreign substance, such as a protein from a pathogen, binds to the B cell receptor to initiate the immune response. Its presence triggers the cascade of events leading to B cell activation and subsequent antibody production.
B cell receptor: Located on the surface of the B cell, this receptor specifically recognizes and binds to the antigen, serving as the first signal for activation. The binding event sets off intracellular signaling pathways that prepare the B cell for further interaction.
B cell: This lymphocyte is responsible for producing antibodies and can differentiate into plasma cells or memory B cells upon activation. Its interaction with antigens and helper T cells is essential for a robust immune response.
Activated B cell: Once signaled by both antigen and T cell cytokines, this B cell enters a state of heightened activity, ready to proliferate and differentiate. It represents a critical step in the transition to antibody secretion and immune memory formation.
MHC II: Major Histocompatibility Complex class II molecules on the B cell present processed antigen fragments to the helper T cell, facilitating recognition. This interaction ensures the T cell can provide the second activation signal to the B cell.
CD4: This co-receptor on the helper T cell enhances the binding to MHC II, stabilizing the interaction between the two cells. It plays a key role in amplifying the T cell’s ability to support B cell activation.
Helper T cell: This T cell subtype provides essential co-stimulation and cytokine signals to the B cell, driving full activation. It bridges innate and adaptive immunity by coordinating the response to T cell-dependent antigens.
T cell receptor: Located on the helper T cell, this receptor recognizes the antigen-MHC II complex, initiating T cell activation. Its engagement is crucial for the T cell to deliver the necessary support to the B cell.
Cytokine: These small proteins, secreted by the helper T cell, act as the second signal to fully activate the B cell. They promote B cell proliferation, differentiation, and antibody class switching, such as from IgM to IgG.
The Mechanism of B and T Cell Collaboration
The interaction between B and T cells is a cornerstone of adaptive immunity. This process ensures a targeted and amplified response to specific pathogens.
- The B cell first encounters an antigen, binding it via its B cell receptor.
- This initial binding provides the first signal, prompting the B cell to process and present the antigen via MHC II.
- The helper T cell recognizes this antigen-MHC II complex through its T cell receptor, aided by CD4.
- Upon recognition, the helper T cell releases cytokines, delivering the second signal to fully activate the B cell.
- Activated B cells then proliferate and differentiate into plasma cells, which secrete antibodies, or memory B cells for future defense.
Role of Dual Signals in B Cell Activation
B cell activation requires two distinct signals for a controlled and effective response. This dual requirement prevents unnecessary immune activation.
- The first signal comes from the antigen binding to the B cell receptor, initiating the process.
- The second signal, provided by cytokines from the helper T cell, ensures the response is specific and amplified.
- This two-signal model reduces the risk of autoimmunity by requiring T cell validation.
- Cytokines also influence antibody class switching, enhancing the immune response’s versatility.
- The interaction highlights the interdependence of B and T cells in mounting a coordinated defense.
Physiological Significance of T Cell-Dependent Antigens
The collaboration between B and T cells has profound implications for immunity. It ensures a robust and adaptable response to complex pathogens.
- T cell-dependent antigens, like those from viruses, require this interaction for full B cell activation.
- Cytokines such as IL-4 and IL-21 drive B cell differentiation into plasma cells and memory B cells.
- This process supports the production of high-affinity antibodies through affinity maturation in germinal centers.
- Memory B cells provide long-term immunity, rapidly responding to re-exposure to the same antigen.
- The system’s efficiency is critical for vaccines, which rely on this mechanism to induce protective immunity.
Clinical Applications and Immune Health
Understanding B and T cell interactions informs therapeutic strategies and vaccine development. This knowledge is vital for enhancing immune responses.
- Vaccines often include adjuvants to mimic T cell help, boosting B cell activation.
- Immunodeficiencies, such as those affecting CD4 T cells in HIV, impair this process, leading to reduced antibody production.
- Monoclonal antibody therapies leverage activated B cells to target specific diseases.
- Cytokine therapies can enhance immune responses in immunocompromised individuals.
- This interplay is key to designing treatments that restore or augment adaptive immunity.
In conclusion, the interaction between B and T cells exemplifies the immune system’s precision and adaptability. By requiring dual signals—antigen binding and cytokine support—the body ensures a tailored response that protects against pathogens while minimizing self-reactivity. This process not only defends against current threats but also builds a foundation for future immunity, underscoring the elegance of immunological cooperation.

