The autonomic nervous system plays a crucial role in regulating involuntary functions like heart rate and blood pressure, with its sympathetic and parasympathetic divisions working in tandem to maintain cardiovascular homeostasis. This detailed diagram illustrates the neural pathways connecting the brainstem and spinal cord to the heart, highlighting how sympathetic fibers and parasympathetic fibers influence cardiac activity through specific ganglia and nerves. Understanding these connections is essential for grasping how the body responds to stress or rest, ensuring efficient blood flow and rhythm control in various physiological states.
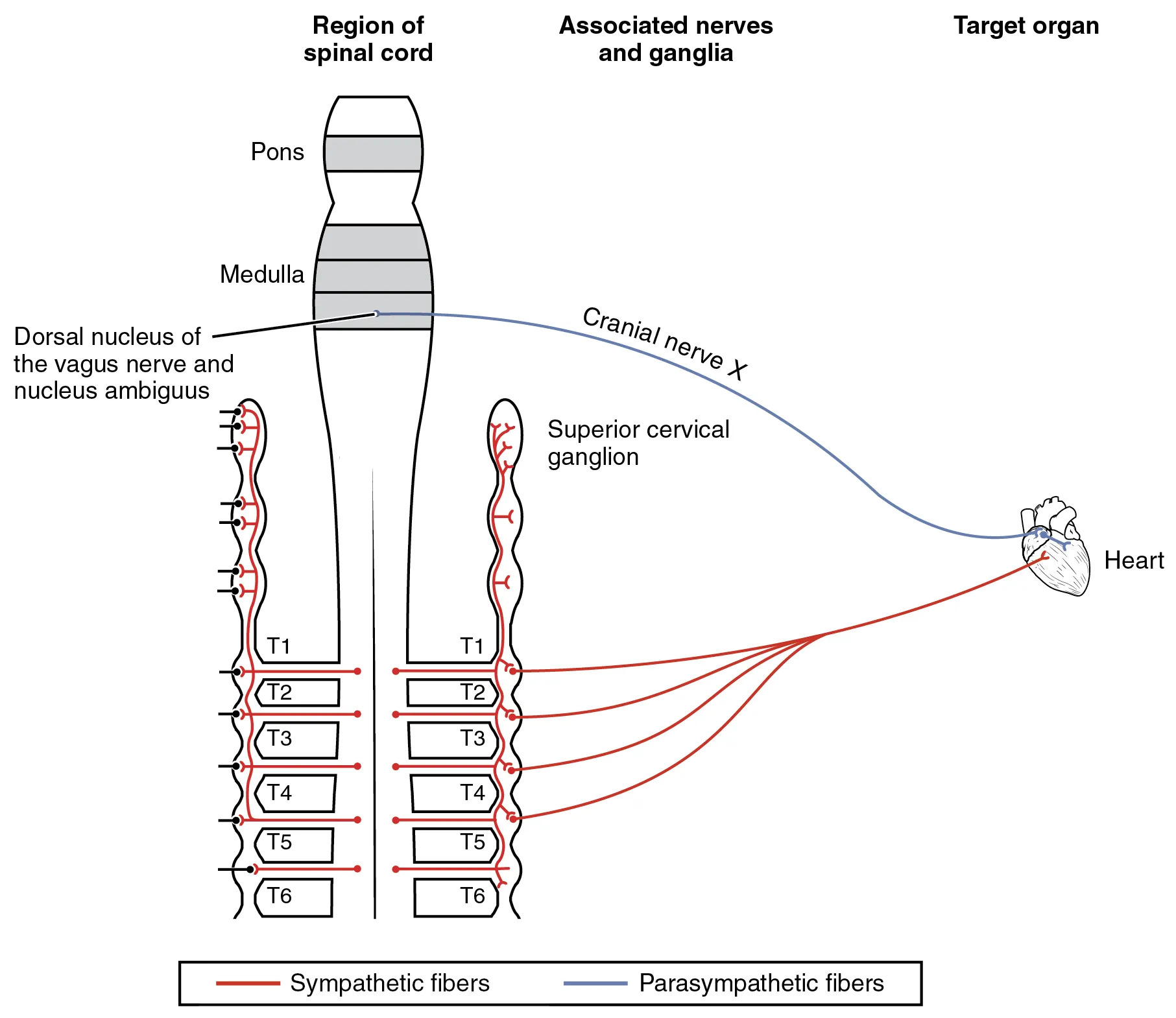
Pons The pons is a key structure in the brainstem that serves as a bridge between higher brain regions and the spinal cord, facilitating communication for motor and sensory pathways. In the context of autonomic control, it contributes to the integration of signals that may indirectly affect cardiovascular functions through its connections with adjacent areas like the medulla.
Medulla The medulla oblongata, located at the base of the brainstem, regulates vital functions such as breathing, heart rate, and blood pressure via its autonomic centers. It houses nuclei that send efferent signals to the heart, making it a central hub for involuntary cardiovascular adjustments in response to internal and external stimuli.
Dorsal nucleus of the vagus nerve and nucleus ambiguus The dorsal nucleus of the vagus nerve is responsible for parasympathetic output to visceral organs, including the heart, where it promotes relaxation and reduced heart rate. The nucleus ambiguus, also involved in vagal control, innervates cardiac muscles to fine-tune rhythm and conduction, ensuring balanced autonomic influence during rest.
Cranial nerve X Cranial nerve X, known as the vagus nerve, carries parasympathetic signals from the brainstem to the heart and other organs, playing a pivotal role in the “rest and digest” response. Its fibers synapse at cardiac ganglia, modulating heart rate by releasing acetylcholine, which slows down pacemaker activity in the sinoatrial node.
Superior cervical ganglion The superior cervical ganglion is part of the sympathetic chain, located near the base of the neck, and relays signals from thoracic spinal segments to the head and heart. It contributes to vasoconstriction and increased heart rate during sympathetic activation, enhancing blood flow to muscles in fight-or-flight scenarios.
T1 T1 refers to the first thoracic spinal cord segment, which gives rise to preganglionic sympathetic neurons that project to paravertebral ganglia. These neurons facilitate cardiovascular acceleration by innervating the heart, increasing contractility and rate to support heightened metabolic demands.
T2 T2, the second thoracic segment, contributes preganglionic fibers to the sympathetic trunk, targeting cardiac accelerators. Its activation leads to enhanced sympathetic tone, which can elevate blood pressure through vasoconstriction in systemic vessels.
T3 T3 involves sympathetic outflow that synapses in chain ganglia before reaching the heart, supporting increased cardiac output. This segment’s role ensures coordinated responses during physical exertion, maintaining adequate perfusion to vital organs.
T4 T4 provides additional sympathetic innervation, with fibers extending to cardiac plexuses for rhythm regulation. It participates in the overall sympathetic drive that counterbalances parasympathetic effects, preventing bradycardia in active states.
T5 T5 contributes to the sympathetic pathways influencing coronary vessel tone and myocardial performance. Its involvement helps in adapting heart function to varying oxygen needs, especially under stress.
T6 T6, the sixth thoracic segment, sends preganglionic axons to ganglia, ultimately affecting heart rate and force of contraction. This level supports sustained sympathetic activity, crucial for long-term cardiovascular adjustments.
Heart The heart serves as the target organ in this diagram, receiving dual innervation that allows for precise control of its pumping action. Autonomic inputs modulate its electrical and mechanical properties, ensuring efficient circulation tailored to bodily needs.
Sympathetic fibers Sympathetic fibers, depicted in red, originate from thoracic spinal levels and accelerate heart rate while strengthening contractions through norepinephrine release. They prepare the body for action by increasing cardiac output and redirecting blood flow.
Parasympathetic fibers Parasympathetic fibers, shown in blue, arise from cranial sources like the vagus nerve, promoting cardiac inhibition to conserve energy during rest. They counteract sympathetic effects, maintaining a balanced heart rhythm.
Region of Spinal cord This section represents the thoracic spinal cord segments involved in autonomic outflow, primarily sympathetic to the heart. It illustrates how neural signals from the central nervous system are organized segmentally for targeted visceral control.
Associated nerves and ganglia This category encompasses the nerves and relay stations like ganglia that transmit autonomic signals to peripheral targets. Ganglia act as synaptic points where preganglionic and postganglionic neurons connect, amplifying or modulating impulses.
Target organ The target organ, here the heart, receives converged autonomic inputs that dictate its functional state. This integration allows for dynamic responses to maintain homeostasis.
Understanding the Autonomic Nervous System’s Role in Cardiovascular Control
The autonomic nervous system divides into sympathetic and parasympathetic branches, each with distinct effects on the heart. This diagram provides a visual roadmap of these pathways, essential for comprehending involuntary regulation.
- The autonomic nervous system operates without conscious effort, influencing heart rate through neurotransmitters like norepinephrine and acetylcholine.
- Sympathetic activation, often triggered by stress, increases heart rate and contractility via beta-adrenergic receptors.
- Parasympathetic dominance, during relaxation, slows the heart by enhancing vagal tone at the sinoatrial node.
- These opposing forces ensure the heart adapts to demands, such as exercise or sleep, preventing extremes in blood pressure.
- Imbalances can lead to conditions like hypertension, underscoring the importance of this neural balance.
Sympathetic Innervation Pathways to the Heart
Sympathetic fibers emerge from the thoracolumbar spinal cord, synapsing in paravertebral ganglia before reaching the heart. This setup allows for widespread activation during emergencies.
- Preganglionic neurons from T1 to T6 exit the spinal cord via ventral roots, entering the sympathetic chain.
- Postganglionic fibers from ganglia like the superior cervical extend to cardiac plexuses, innervating atria and ventricles.
- Norepinephrine binds to beta-1 receptors, elevating cyclic AMP levels to boost pacemaker activity.
- This pathway enhances stroke volume, crucial for oxygen delivery during physical activity.
- Chronic sympathetic overactivity may contribute to cardiac remodeling, affecting long-term heart health.
Parasympathetic Innervation and Vagal Influence
Parasympathetic signals originate in the craniosacral regions, with the vagus nerve as the primary conduit to the heart. This branch promotes recovery and energy conservation.
- The dorsal vagal nucleus and nucleus ambiguus in the medulla generate preganglionic fibers that travel via cranial nerve X.
- These fibers synapse at intracardiac ganglia, releasing acetylcholine to activate muscarinic receptors.
- Activation hyperpolarizes cells, reducing firing rate in the atrioventricular node for controlled conduction.
- Vagal stimulation can induce bradycardia, beneficial in countering tachyarrhythmias.
- Interactions with baroreceptors help regulate blood pressure reflexes, maintaining stability.
Integration of Autonomic Signals at the Heart
At the cardiac level, sympathetic and parasympathetic inputs converge on pacemaker cells and myocardium. This dual control prevents erratic function, as seen in the diagram’s depiction.
- The sinoatrial node, the heart’s primary pacemaker, receives dense innervation from both systems.
- Sympathetic fibers increase slope of phase 4 depolarization, accelerating rhythm.
- Parasympathetic effects open potassium channels, prolonging repolarization for slower rates.
- This antagonism allows fine-tuning, where nicotine’s impact on ganglia could disrupt balance, potentially causing arrhythmias.
- Blood vessels also receive sympathetic vasoconstrictor tones, with no direct parasympathetic counterpart for systemic pressure.
Physiological Implications of Autonomic Control
Autonomic regulation extends beyond the heart to influence overall circulation and organ perfusion. The diagram highlights how these nerves maintain equilibrium.
- During fight-or-flight, sympathetic dominance shunts blood from viscera to muscles, raising pressure.
- Rest-and-digest states enhance parasympathetic activity, promoting digestion while slowing cardiac output.
- Hormonal interactions, such as epinephrine from adrenals, amplify sympathetic effects on the heart.
- Thyroid hormones like T3 and T4 sensitize cardiac tissues to catecholamines, modulating basal metabolic rate.
- Disruptions, like in autonomic neuropathies, can impair these responses, leading to orthostatic hypotension.
Clinical Relevance of the Diagram
This illustration serves as a foundational tool for understanding cardiovascular autonomic disorders. It bridges anatomy with function, aiding in diagnostic approaches.
- In conditions like heart failure, sympathetic hyperactivity compensates but may exacerbate damage over time.
- Vagal nerve stimulation therapies target parasympathetic pathways to treat resistant hypertension or arrhythmias.
- Pharmacological agents, such as beta-blockers, mimic or block these neural effects for therapeutic control.
- Imaging techniques, like MRI of the brainstem, can reveal lesions affecting medullary nuclei.
- Educational value lies in visualizing how nicotine affects ganglia without mutual cancellation, leading to hypertension.
Future Directions in Autonomic Research
Advancements in neuroscience continue to unravel complexities in cardiac innervation. This diagram inspires ongoing studies into targeted interventions.
- Neuromodulation devices aim to selectively activate vagal fibers for anti-inflammatory effects on the heart.
- Genetic studies explore variations in receptor expression influencing autonomic responsiveness.
- Bioengineering models simulate these pathways to test drug efficacy without human trials.
- Integration with wearable tech monitors autonomic balance in real-time for preventive care.
- Research into autonomic plasticity post-injury offers hope for regenerating damaged pathways.
In summary, this diagram of autonomic connections to the heart encapsulates the intricate balance between sympathetic and parasympathetic systems, vital for cardiovascular health. By detailing neural origins, pathways, and effects, it provides a clear framework for appreciating how the body sustains life through unconscious regulation, paving the way for informed clinical and research pursuits.

