Explore the vital process of fatty acid oxidation, also known as beta-oxidation, a key metabolic pathway that converts fatty acids into acetyl CoA for energy production. This crucial mechanism ensures the body has an ample fuel supply, especially during periods of low glucose availability or prolonged physical activity.
Understanding the Breakdown of Fatty Acids Diagram
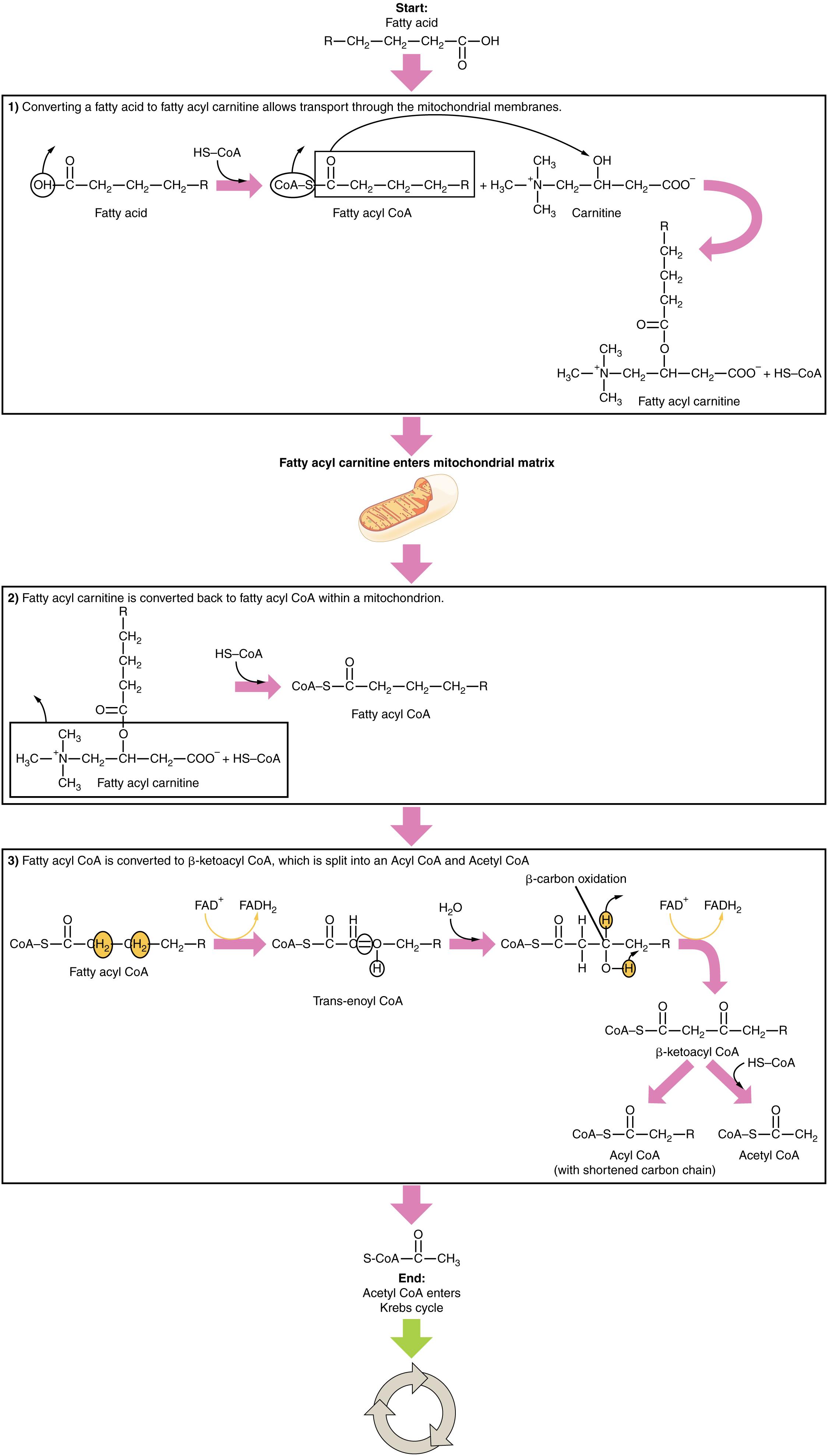
Start: Fatty acid: Fatty acids are carboxylic acids with a long aliphatic chain, either saturated or unsaturated. They serve as a major energy source for the body, particularly when glucose is scarce, and are the building blocks of lipids like triglycerides.
HS-CoA: Coenzyme A, a vital coenzyme, plays a crucial role in the metabolism of fatty acids, carbohydrates, and proteins. In fatty acid activation, HS-CoA combines with the fatty acid to form fatty acyl CoA, preparing it for mitochondrial entry and subsequent oxidation.
Fatty acyl CoA: This is an activated fatty acid molecule, formed by the esterification of a fatty acid with coenzyme A. The formation of fatty acyl CoA is an ATP-dependent reaction that makes the fatty acid reactive for transport and metabolic processes.
Carnitine: A quaternary ammonium compound that is essential for the transport of long-chain fatty acids from the cytosol into the mitochondrial matrix. Carnitine temporarily binds to fatty acyl CoA to form fatty acyl carnitine, allowing it to cross the inner mitochondrial membrane.
Fatty acyl carnitine: This molecule is formed when fatty acyl CoA combines with carnitine. Its formation is a critical step in the carnitine shuttle system, enabling long-chain fatty acids to enter the mitochondria for beta-oxidation.
Fatty acyl carnitine enters mitochondrial matrix: This represents the active transport of fatty acyl carnitine from the intermembrane space into the mitochondrial matrix. This transport is mediated by carnitine-acylcarnitine translocase, a protein embedded in the inner mitochondrial membrane.
Fatty acyl CoA is converted to β-ketoacyl CoA, which is split into an acyl CoA and Acetyl CoA: This describes the core process of beta-oxidation, where a fatty acyl CoA undergoes a series of four reactions. Each cycle shortens the fatty acid chain by two carbon atoms, producing one molecule of acetyl CoA, one NADH, and one FADH2.
FAD+: Flavin adenine dinucleotide, in its oxidized form, acts as an electron acceptor in the first oxidative step of beta-oxidation. It is reduced to FADH2, carrying high-energy electrons to the electron transport chain.
FADH2: The reduced form of FAD+, carrying high-energy electrons generated during the oxidation of fatty acyl CoA. FADH2 will donate these electrons to the electron transport chain, contributing to ATP production.
Trans-enoyl CoA: An intermediate formed during beta-oxidation, specifically after the first dehydrogenation step catalyzed by acyl-CoA dehydrogenase. It features a trans double bond between the alpha and beta carbons of the fatty acyl chain.
H2O: Water is consumed during the hydration step of beta-oxidation, where it is added across the double bond of trans-enoyl CoA. This reaction forms L-β-hydroxyacyl CoA, preparing the molecule for the next oxidative step.
NAD+: Nicotinamide adenine dinucleotide, in its oxidized form, acts as an electron acceptor in the third oxidative step of beta-oxidation. It is reduced to NADH, carrying high-energy electrons to the electron transport chain.
NADH: The reduced form of NAD+, carrying high-energy electrons generated during the oxidation of L-β-hydroxyacyl CoA. NADH will donate these electrons to the electron transport chain, significantly contributing to ATP production.
β-ketoacyl CoA: This intermediate is formed after the second oxidative step of beta-oxidation. It contains a keto group on the beta-carbon, making it susceptible to cleavage by thiolase.
Acetyl CoA (with shortened carbon chain): After one cycle of beta-oxidation, one acetyl CoA molecule is released, and the remaining fatty acyl CoA is shortened by two carbon atoms. This shortened fatty acyl CoA then re-enters the beta-oxidation pathway for further rounds of oxidation.
Acetyl CoA enters Krebs cycle: The final destination for the acetyl CoA molecules produced from fatty acid oxidation. Acetyl CoA enters the Krebs cycle, where it is completely oxidized, generating more NADH, FADH2, and ATP.
Krebs cycle: The central metabolic pathway where acetyl CoA is oxidized to carbon dioxide, producing significant amounts of NADH and FADH2. These reduced coenzymes then fuel the electron transport chain for massive ATP generation.
The breakdown of fatty acids, primarily through a process known as beta-oxidation, is a crucial metabolic pathway that allows the body to harness the substantial energy stored in lipids. While glucose is the preferred fuel source for many tissues, fatty acids become increasingly important during periods of fasting, prolonged exercise, or when carbohydrate availability is low. This intricate catabolic process occurs predominantly within the mitochondria, systematically dismantling long-chain fatty acids into two-carbon acetyl CoA units, which can then enter the Krebs cycle to generate ATP.
Fatty acids are incredibly energy-dense molecules; their complete oxidation yields significantly more ATP per carbon atom than carbohydrates. This makes them an invaluable fuel reserve, particularly for organs like the heart and skeletal muscles, which can readily utilize fatty acids for continuous energy production. However, before fatty acids can be oxidized, they must first be activated and then transported into the mitochondrial matrix, where beta-oxidation takes place. This initial preparatory phase is essential to make the hydrophobic fatty acids amenable to enzymatic reactions within the aqueous environment of the cell.
The activation of a fatty acid involves its attachment to coenzyme A, forming a fatty acyl CoA molecule, an ATP-dependent reaction that occurs in the cytosol. Long-chain fatty acyl CoA molecules then require a specialized shuttle system, known as the carnitine shuttle, to cross the inner mitochondrial membrane, which is otherwise impermeable to them. Once inside the mitochondrial matrix, the fatty acyl CoA undergoes successive cycles of beta-oxidation. Each cycle involves four distinct enzymatic steps that result in the removal of two carbon atoms in the form of acetyl CoA, along with the production of one NADH and one FADH2 molecule.
- Fatty acid oxidation is known as beta-oxidation.
- It occurs primarily in the mitochondria.
- It produces acetyl CoA, NADH, and FADH2.
- It is a major energy source during fasting and low glucose.
The Mechanism of Beta-Oxidation
The carnitine shuttle is a sophisticated transport system essential for moving long-chain fatty acids into the mitochondrial matrix. After a fatty acid is activated to fatty acyl CoA in the cytosol, carnitine palmitoyltransferase I (CPT-I), located on the outer mitochondrial membrane, catalyzes the transfer of the fatty acyl group from CoA to carnitine, forming fatty acyl carnitine. This fatty acyl carnitine then moves across the inner mitochondrial membrane via carnitine-acylcarnitine translocase. Once inside the matrix, carnitine palmitoyltransferase II (CPT-II) reverses the reaction, reforming fatty acyl CoA and releasing free carnitine, which is then recycled back to the cytosol.
Once inside the mitochondrial matrix, the fatty acyl CoA undergoes beta-oxidation. Each cycle of beta-oxidation consists of four enzymatic reactions:
- Dehydrogenation: Acyl-CoA dehydrogenase oxidizes the fatty acyl CoA, forming a trans-enoyl CoA and producing FADH2. This enzyme creates a double bond between the alpha and beta carbons.
- Hydration: Enoyl-CoA hydratase adds water across the double bond of the trans-enoyl CoA, forming L-β-hydroxyacyl CoA. This step prepares the molecule for the next oxidation.
- Dehydrogenation: L-β-hydroxyacyl CoA dehydrogenase oxidizes the hydroxyl group on the beta-carbon, forming β-ketoacyl CoA and producing NADH. This is the second oxidative step in the cycle.
- Thiolytic cleavage: Thiolase catalyzes the cleavage of the β-ketoacyl CoA, releasing one molecule of acetyl CoA and a fatty acyl CoA that is two carbons shorter than the original.
This shortened fatty acyl CoA then re-enters the beta-oxidation pathway, undergoing successive rounds until the entire fatty acid chain is converted into acetyl CoA units. For example, a 16-carbon palmitic acid would undergo seven cycles of beta-oxidation, producing eight molecules of acetyl CoA, seven FADH2, and seven NADH. Each of these products (acetyl CoA, NADH, FADH2) then feeds into other major energy-producing pathways.
Energy Yield and Clinical Significance
The acetyl CoA generated from beta-oxidation is fed directly into the Krebs cycle, where it is further oxidized to produce more NADH and FADH2. These electron carriers, along with those produced during beta-oxidation itself, then enter the electron transport chain to drive the synthesis of a large amount of ATP through oxidative phosphorylation. The complete oxidation of a single palmitic acid molecule (16 carbons) yields approximately 106 molecules of ATP, highlighting the significant energy contribution of fatty acids compared to glucose.
Disorders of fatty acid oxidation can have severe clinical implications, particularly for tissues highly dependent on fat metabolism, such as the heart and skeletal muscles, and during periods of fasting when the body relies on fatty acids for fuel. Conditions like medium-chain acyl-CoA dehydrogenase (MCAD) deficiency are among the most common inherited metabolic disorders. Individuals with MCAD deficiency cannot effectively break down medium-chain fatty acids, leading to an accumulation of toxic intermediates and impaired energy production, especially during fasting. Symptoms can include hypoglycemia, lethargy, muscle weakness, and in severe cases, coma or death. Early diagnosis through newborn screening and dietary management (avoiding prolonged fasting) are crucial for these patients.
Other disorders can affect the carnitine shuttle system or other enzymes involved in beta-oxidation, leading to similar symptoms. Understanding the molecular mechanisms of fatty acid breakdown is therefore critical for the diagnosis, treatment, and management of these life-threatening metabolic conditions, ensuring that affected individuals receive appropriate medical care to prevent serious complications.
Conclusion
The breakdown of fatty acids through beta-oxidation is a meticulously regulated and highly efficient process, providing a substantial source of energy for the body, particularly when glucose reserves are low. From the initial activation and carnitine-mediated transport into the mitochondria to the cyclical cleavage into acetyl CoA, this pathway is fundamental to cellular energy homeostasis. Its intricate steps culminate in the generation of vast amounts of ATP, underscoring its vital role in human physiology and its profound implications for health when disruptions occur.

