Explore gluconeogenesis, a vital metabolic pathway that synthesizes glucose from non-carbohydrate precursors, ensuring a steady supply of energy for glucose-dependent organs. This intricate process is essential during fasting or prolonged exercise, playing a critical role in maintaining blood glucose homeostasis.
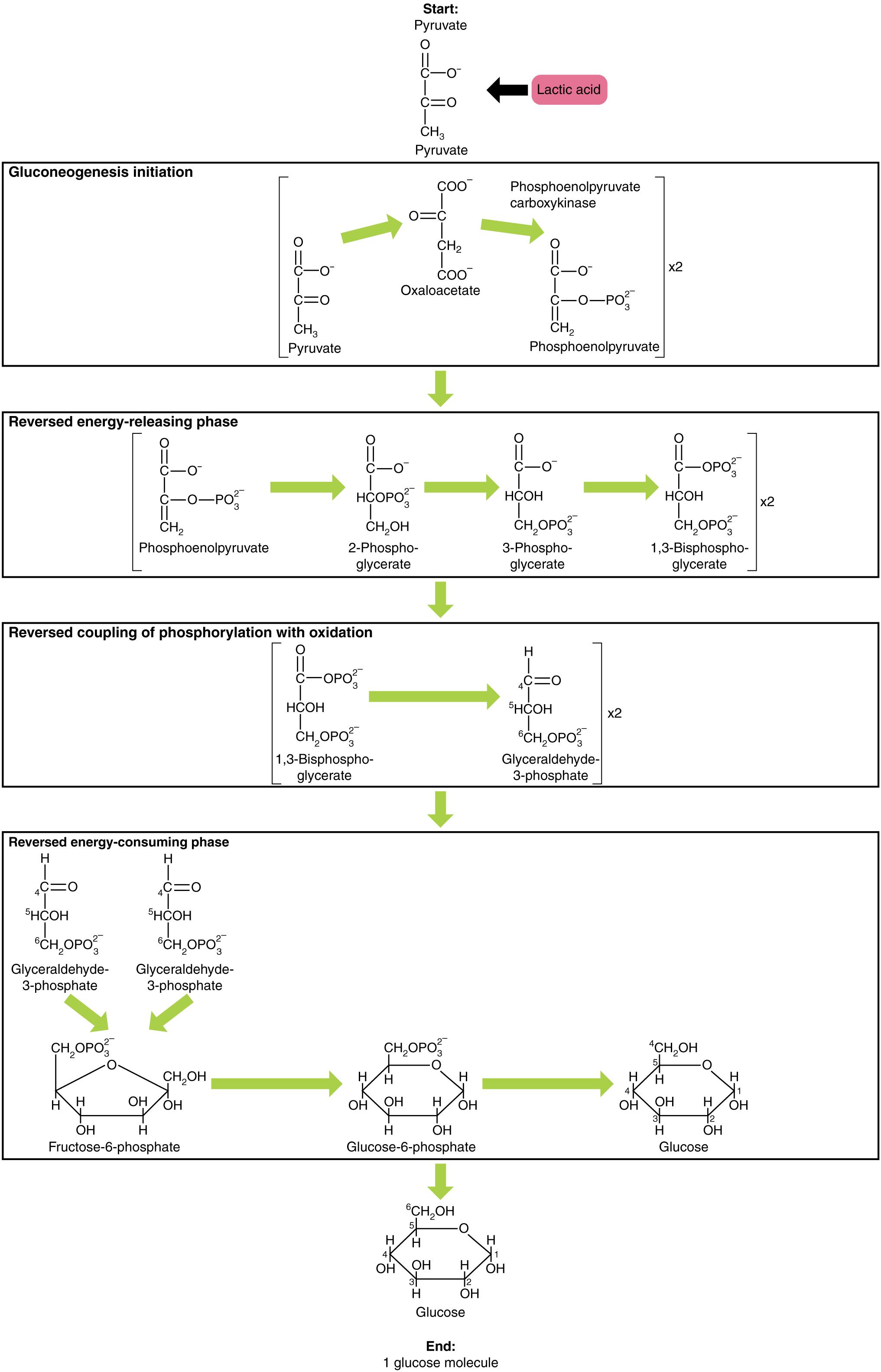
Understanding the Gluconeogenesis Diagram
Start: Pyruvate: Pyruvate is a three-carbon alpha-keto acid that serves as a primary starting material for gluconeogenesis. It is derived from glycolysis, amino acid metabolism, or lactate, marking a crucial entry point into the glucose synthesis pathway.
Lactic acid: Lactic acid (lactate) is a common precursor for gluconeogenesis, particularly during intense exercise when oxygen supply is limited. It is converted back to pyruvate, which then enters the gluconeogenic pathway to produce glucose.
Gluconeogenesis initiation: This phase involves the conversion of pyruvate into phosphoenolpyruvate (PEP), circumventing the irreversible pyruvate kinase step of glycolysis. This set of reactions requires energy and often occurs across both mitochondrial and cytosolic compartments.
Phosphoenolpyruvate carboxykinase: This enzyme is a key regulatory point in gluconeogenesis, catalyzing the conversion of oxaloacetate to phosphoenolpyruvate (PEP). It utilizes GTP as an energy source and is crucial for overcoming the irreversible pyruvate kinase step of glycolysis.
Oxaloacetate: A four-carbon dicarboxylic acid that is an intermediate in the conversion of pyruvate to phosphoenolpyruvate during gluconeogenesis. It is formed from pyruvate within the mitochondria before being transported to the cytoplasm for further steps.
Phosphoenolpyruvate (PEP): This high-energy phosphate compound is a critical intermediate in both glycolysis and gluconeogenesis. In gluconeogenesis, it is formed from oxaloacetate and is then metabolized through a series of reversible steps to fructose-1,6-bisphosphate.
Reversed energy-releasing phase: This refers to the reversal of glycolytic steps that normally release energy. In gluconeogenesis, these reactions are driven in the opposite direction, utilizing ATP and NADH to build up glucose.
2-Phosphoglycerate: An intermediate in both glycolysis and gluconeogenesis, this three-carbon molecule is isomerized from 3-phosphoglycerate. Its formation is part of the reversible steps linking PEP to the triose phosphates.
3-Phosphoglycerate: This three-carbon molecule is an intermediate in gluconeogenesis, formed from 1,3-bisphosphoglycerate. It is subsequently isomerized to 2-phosphoglycerate as the pathway progresses towards glucose synthesis.
1,3-Bisphosphoglycerate: This high-energy intermediate is formed from 3-phosphoglycerate through an ATP-dependent phosphorylation in gluconeogenesis (reversing the glycolytic step where it’s formed from glyceraldehyde-3-phosphate). It is a crucial point for energy investment in the synthesis direction.
Reversed coupling of phosphorylation with oxidation: This describes the reversal of the glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase reactions. In gluconeogenesis, these steps consume ATP and NADH to build up the carbon backbone.
Glyceraldehyde-3-phosphate: A three-carbon aldehyde that is a key intermediate in gluconeogenesis, formed from 1,3-bisphosphoglycerate. It represents the splitting of hexose molecules into two triose phosphates, which are then combined to form fructose-1,6-bisphosphate.
Reversed energy-consuming phase: This represents the final set of steps in gluconeogenesis that are the reversal of the energy-consuming steps of glycolysis. These steps overcome irreversible glycolytic reactions using specific enzymes to produce glucose.
Fructose-6-phosphate: This six-carbon phosphorylated sugar is an intermediate in gluconeogenesis, formed from fructose-1,6-bisphosphate by the enzyme fructose-1,6-bisphosphatase. It is a precursor to glucose-6-phosphate.
Glucose-6-phosphate: A six-carbon phosphorylated glucose molecule that is a crucial intermediate in gluconeogenesis. It is dephosphorylated by glucose-6-phosphatase to release free glucose, primarily in the liver.
Glucose: The final product of gluconeogenesis, a six-carbon sugar that is the primary energy source for many cells, especially those of the brain and red blood cells. Its synthesis is vital for maintaining blood glucose levels during periods of fasting or low carbohydrate intake.
End: 1 glucose molecule: This signifies the successful completion of the gluconeogenesis pathway, culminating in the production of one molecule of glucose. This newly synthesized glucose can then be released into the bloodstream to supply other tissues.
Gluconeogenesis is a pivotal metabolic pathway that ensures the body’s continued supply of glucose, particularly during periods of fasting, prolonged strenuous exercise, or low carbohydrate intake. Unlike glycolysis, which breaks down glucose, gluconeogenesis is the anabolic process of synthesizing glucose from non-carbohydrate precursors. These precursors can include lactate, derived from anaerobic glycolysis in muscle; amino acids, released from protein breakdown; and glycerol, a component of triglycerides. This vital process primarily occurs in the liver, with a smaller contribution from the kidneys, acting as a critical mechanism to maintain blood glucose homeostasis.
The necessity for gluconeogenesis stems from the fact that certain tissues, most notably the brain and red blood cells, have an obligate requirement for glucose as their primary energy source. While glycogen stores provide a readily available supply of glucose for a limited time (typically 12-18 hours), these reserves can become depleted. During prolonged fasting or starvation, gluconeogenesis becomes the sole pathway for de novo glucose synthesis, preventing hypoglycemia and ensuring the survival of glucose-dependent organs. Without this adaptive pathway, the body would quickly run out of essential fuel, leading to severe physiological dysfunction.
Gluconeogenesis is not simply the reverse of glycolysis; it involves unique bypass reactions to overcome the three irreversible steps of glycolysis. These bypasses are catalyzed by specific enzymes that ensure the pathway is energetically favorable in the direction of glucose synthesis. For instance, pyruvate is converted to phosphoenolpyruvate (PEP) via oxaloacetate, bypassing the pyruvate kinase step. Similarly, fructose-1,6-bisphosphatase and glucose-6-phosphatase catalyze the dephosphorylation steps that reverse the irreversible phosphofructokinase-1 and hexokinase reactions of glycolysis, respectively. This intricate regulation ensures that glycolysis and gluconeogenesis do not operate simultaneously in a futile cycle.
-
Gluconeogenesis synthesizes glucose from non-carbohydrate precursors.
-
It primarily occurs in the liver and kidneys.
-
It is crucial for maintaining blood glucose levels during fasting.
-
It utilizes unique bypass reactions to reverse irreversible glycolytic steps.
The Mechanism of Glucose Synthesis
The initiation of gluconeogenesis typically begins with pyruvate, which is transported into the mitochondrial matrix. Inside the mitochondrion, pyruvate is carboxylated to oxaloacetate by the enzyme pyruvate carboxylase, a reaction that requires ATP and carbon dioxide. Oxaloacetate, a four-carbon molecule, cannot directly cross the mitochondrial membrane. Therefore, it is converted to malate (or aspartate) to exit the mitochondrion and then reconverted back to oxaloacetate in the cytoplasm. Once in the cytoplasm, oxaloacetate is decarboxylated and phosphorylated to phosphoenolpyruvate (PEP) by the enzyme phosphoenolpyruvate carboxykinase (PEPCK), a reaction that consumes GTP.
From phosphoenolpyruvate, the pathway largely follows a reversal of glycolytic steps, but in the opposite direction. A series of reversible reactions convert PEP back to fructose-1,6-bisphosphate. However, to bypass the irreversible phosphofructokinase-1 reaction of glycolysis, gluconeogenesis utilizes fructose-1,6-bisphosphatase. This enzyme removes a phosphate group from fructose-1,6-bisphosphate to form fructose-6-phosphate, requiring no ATP but releasing inorganic phosphate. This step is a key regulatory point, ensuring the directional flow of carbon towards glucose synthesis.
The next irreversible glycolytic step that must be bypassed is the hexokinase reaction, which converts glucose to glucose-6-phosphate. In gluconeogenesis, fructose-6-phosphate is isomerized to glucose-6-phosphate. Subsequently, glucose-6-phosphatase, an enzyme predominantly found in the endoplasmic reticulum of liver and kidney cells, dephosphorylates glucose-6-phosphate to release free glucose. This final step is crucial because it allows the newly synthesized glucose to be transported out of the cell and into the bloodstream, where it can be utilized by glucose-dependent tissues.
Clinical Significance of Gluconeogenesis
The precise regulation of gluconeogenesis is vital for maintaining metabolic health, and dysregulation can lead to significant clinical consequences. In conditions like Type 2 diabetes mellitus, an overactive gluconeogenesis in the liver contributes significantly to hyperglycemia, particularly in the fasting state. Despite elevated blood glucose levels, the liver continues to produce glucose, exacerbating the problem. This sustained hepatic glucose output, coupled with insulin resistance in peripheral tissues, creates a vicious cycle that contributes to the chronic high blood sugar characteristic of diabetes.
Conversely, defects in gluconeogenic enzymes can lead to severe hypoglycemia, especially during periods of fasting. For example, inherited deficiencies in enzymes like glucose-6-phosphatase (causing Glycogen Storage Disease Type I, or Von Gierke’s disease) or fructose-1,6-bisphosphatase result in the inability to produce glucose from non-carbohydrate precursors. Patients with these conditions require strict dietary management, including frequent carbohydrate intake, to prevent life-threatening drops in blood sugar. Understanding the regulatory mechanisms and enzymatic steps of gluconeogenesis is therefore paramount for diagnosing and managing these metabolic disorders.
Conclusion
Gluconeogenesis stands as a testament to the body’s remarkable adaptive capacity, ensuring a continuous supply of glucose for vital organs even in the absence of dietary carbohydrates. This complex anabolic pathway, primarily centered in the liver, skillfully reverses elements of glycolysis and bypasses irreversible steps to synthesize glucose from precursors like lactate, amino acids, and glycerol. Its meticulous regulation is paramount for maintaining blood glucose homeostasis, and dysfunctions within this pathway are implicated in significant metabolic diseases, highlighting its critical importance in human physiology and health.

