Explore the crucial mechanisms of carbon dioxide transport in the blood, essential for removing this metabolic waste product from tissues and delivering it to the lungs for exhalation. This article details the three primary methods: transport in red blood cells, as bicarbonate ions in plasma, and dissolved directly in plasma.
The Vital Journey: How Your Body Manages Carbon Dioxide
Carbon dioxide (CO2) is a metabolic waste product continuously generated by cells throughout the body during cellular respiration. While essential for life, the accumulation of CO2 can significantly alter blood pH, leading to acidosis and compromising vital physiological functions. Therefore, the efficient transport of carbon dioxide from the tissues, where it is produced, to the lungs, where it can be exhaled, is a critical component of respiratory and circulatory health.
This complex journey involves multiple sophisticated mechanisms, ensuring that CO2 is effectively carried through the bloodstream despite its relatively low solubility in plasma. The human body employs three primary methods to transport CO2, each contributing to the overall efficiency of waste removal and acid-base balance. Understanding these pathways is fundamental to comprehending respiratory physiology and diagnosing conditions related to CO2 retention or elimination.
The three main methods of CO2 transport are:
- As bicarbonate ions in the plasma.
- Bound to hemoglobin within red blood cells.
- Dissolved directly in the plasma.
These mechanisms work in concert to maintain physiological homeostasis.
The Three Pillars of Carbon Dioxide Transport
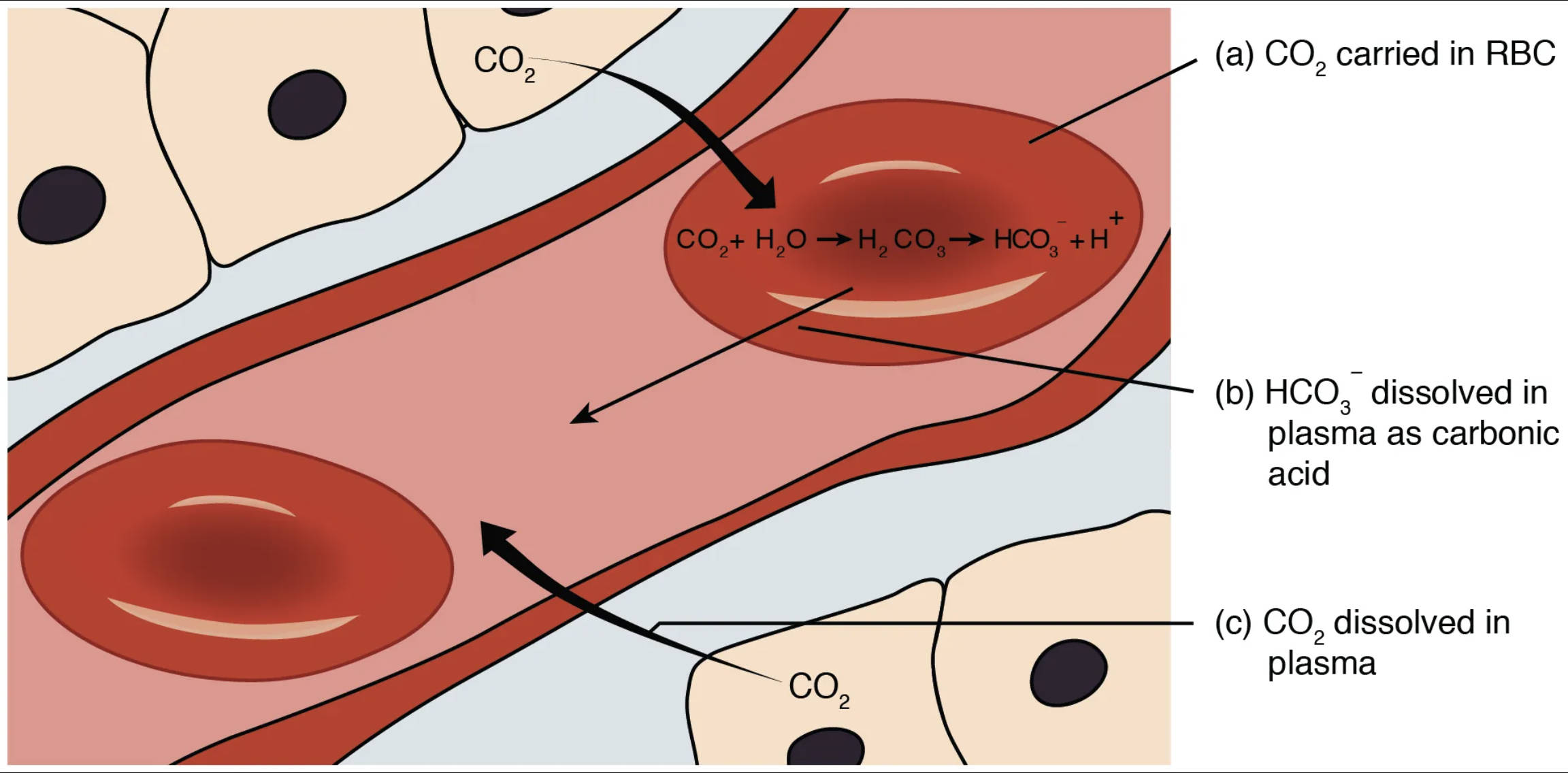
The image clearly illustrates the three principal ways carbon dioxide is transported from the body’s tissues back to the lungs. This multi-faceted approach ensures efficient removal of this metabolic waste product.
CO2 (carbon dioxide): This represents the carbon dioxide molecules diffusing from the tissue cells into the capillaries. CO2 is a byproduct of cellular metabolism and must be efficiently transported away from the tissues to prevent its accumulation and maintain pH balance.
(a) CO2 carried in RBC: This method refers to carbon dioxide being transported within the red blood cells, primarily bound to hemoglobin (forming carbaminohemoglobin) or rapidly converted into bicarbonate ions. Red blood cells play a central role in CO2 transport.
CO2 + H2O ⇌ H2CO3 ⇌ HCO3- + H+: This chemical reaction occurs predominantly within red blood cells. Carbon dioxide (CO2) rapidly combines with water (H2O) to form carbonic acid (H2CO3), a reaction catalyzed by the enzyme carbonic anhydrase. Carbonic acid then quickly dissociates into a hydrogen ion (H+) and a bicarbonate ion (HCO3-). This reversible reaction is crucial for the efficient conversion and transport of CO2.
(b) HCO3- dissolved in plasma as carbonic acid: This refers to the primary method of CO2 transport, where bicarbonate ions (HCO3-), predominantly formed inside red blood cells, diffuse into the blood plasma. These bicarbonate ions are then transported in the plasma to the lungs, where the reaction reverses, and CO2 is released for exhalation.
(c) CO2 dissolved in plasma: This indicates that a small percentage of carbon dioxide is transported directly dissolved in the blood plasma. While a minor contributor to overall CO2 transport, this dissolved CO2 contributes to the partial pressure gradient that drives its movement.
Delving Deeper into CO2 Transport Mechanisms
Each of the three mechanisms for carbon dioxide transport plays a distinct yet interconnected role in maintaining the body’s acid-base balance and facilitating gas exchange.
1. Dissolved in Plasma (Approximately 7-10%)
The simplest form of CO2 transport involves a small percentage of carbon dioxide molecules dissolving directly into the blood plasma. Due to CO2’s relatively low solubility in water, this method accounts for only a minor fraction of the total CO2 transported. However, the dissolved CO2 is crucial because it contributes to the partial pressure of carbon dioxide (PCO2) in the blood. This PCO2 gradient is the driving force that facilitates the diffusion of CO2 from the tissues into the blood and, subsequently, from the blood into the alveoli for exhalation.
2. Bound to Hemoglobin (Carbaminohemoglobin) (Approximately 20-30%)
Within the red blood cells, carbon dioxide can bind directly to the amino groups of hemoglobin (Hb), forming a compound called carbaminohemoglobin (CO2 + Hb ⇌ HbCO2). This binding does not occur at the same binding sites as oxygen, meaning CO2 and O2 can be transported simultaneously, though they influence each other’s binding affinity (Haldane effect). The formation of carbaminohemoglobin is favored when hemoglobin is deoxygenated (as in the tissues), and its dissociation is favored when hemoglobin binds oxygen (as in the lungs), further enhancing the efficiency of gas exchange.
3. As Bicarbonate Ions in Plasma (Approximately 70%)
This is the most significant method of carbon dioxide transport and involves a crucial enzymatic reaction occurring primarily within red blood cells. As CO2 diffuses into red blood cells from the tissues, it rapidly combines with water (H2O) to form carbonic acid (H2CO3). This reaction is powerfully catalyzed by the enzyme carbonic anhydrase, which speeds it up by several thousand-fold (CO2 + H2O ⇌ H2CO3). Carbonic acid then quickly dissociates into a hydrogen ion (H+) and a bicarbonate ion (HCO3-). Most of these bicarbonate ions then diffuse out of the red blood cell and into the blood plasma, in exchange for chloride ions (the “chloride shift”), to be transported to the lungs. The hydrogen ions, being highly acidic, are buffered by binding to deoxyhemoglobin, preventing a drastic drop in blood pH. In the lungs, this entire process reverses, allowing CO2 to be reformed and exhaled.
Clinical Relevance of CO2 Transport
Disruptions in carbon dioxide transport can have profound clinical implications. Conditions that impair CO2 removal from the body, such as hypoventilation (insufficient breathing), can lead to hypercapnia (elevated CO2 levels in the blood) and respiratory acidosis. Conversely, excessive CO2 elimination, as seen in hyperventilation, can result in hypocapnia and respiratory alkalosis. Both acidosis and alkalosis can disrupt enzyme function, alter electrolyte balance, and severely impact the nervous system, highlighting the critical role of efficient CO2 transport in maintaining physiological homeostasis. Medical interventions often target these transport mechanisms to manage acid-base disorders in patients.
Conclusion
The sophisticated mechanisms of carbon dioxide transport—dissolved in plasma, bound as carbaminohemoglobin, and primarily as bicarbonate ions—collectively ensure the efficient removal of this metabolic waste product from the tissues and its delivery to the lungs for exhalation. This intricate physiological process is fundamental not only for maintaining optimal cellular function but also for regulating the delicate acid-base balance of the blood. A comprehensive understanding of these transport pathways is indispensable for medical professionals in diagnosing and managing a wide array of respiratory and metabolic conditions, ultimately contributing to improved patient outcomes and overall health.

