Explore the vital connection between blood pH and oxygen delivery to tissues, as illustrated by the effect of pH on the oxygen-hemoglobin dissociation curve. This article delves into the Bohr effect, explaining how changes in acidity optimize oxygen release to metabolically active cells, a crucial aspect of respiratory physiology.
The Dynamic Duo: pH and Oxygen Transport
The efficient transport of oxygen throughout the body is not solely dependent on the partial pressure of oxygen (PO2); it is also intricately modulated by various physiological factors, chief among them being blood pH. The relationship between pH and oxygen-hemoglobin binding affinity is a cornerstone of respiratory physiology, enabling the body to precisely regulate oxygen delivery to tissues based on their metabolic needs. This dynamic interplay is graphically represented by shifts in the oxygen-hemoglobin dissociation curve.
Understanding how pH influences this curve, a phenomenon known as the Bohr effect, is crucial for comprehending how the body optimizes oxygen release to actively metabolizing cells while ensuring efficient oxygen loading in the lungs. Deviations in blood pH, which can occur in various disease states, significantly impact oxygen availability at the tissue level, underscoring the medical importance of this physiological mechanism.
Key aspects of the pH effect on oxygen transport include:
- Optimal oxygen release in acidic, active tissues.
- Enhanced oxygen binding in alkaline, lung environments.
- A physiological adaptation to metabolic demand.
These mechanisms highlight the body’s sophisticated control over oxygen homeostasis.
The Bohr Effect: pH and Hemoglobin’s Affinity
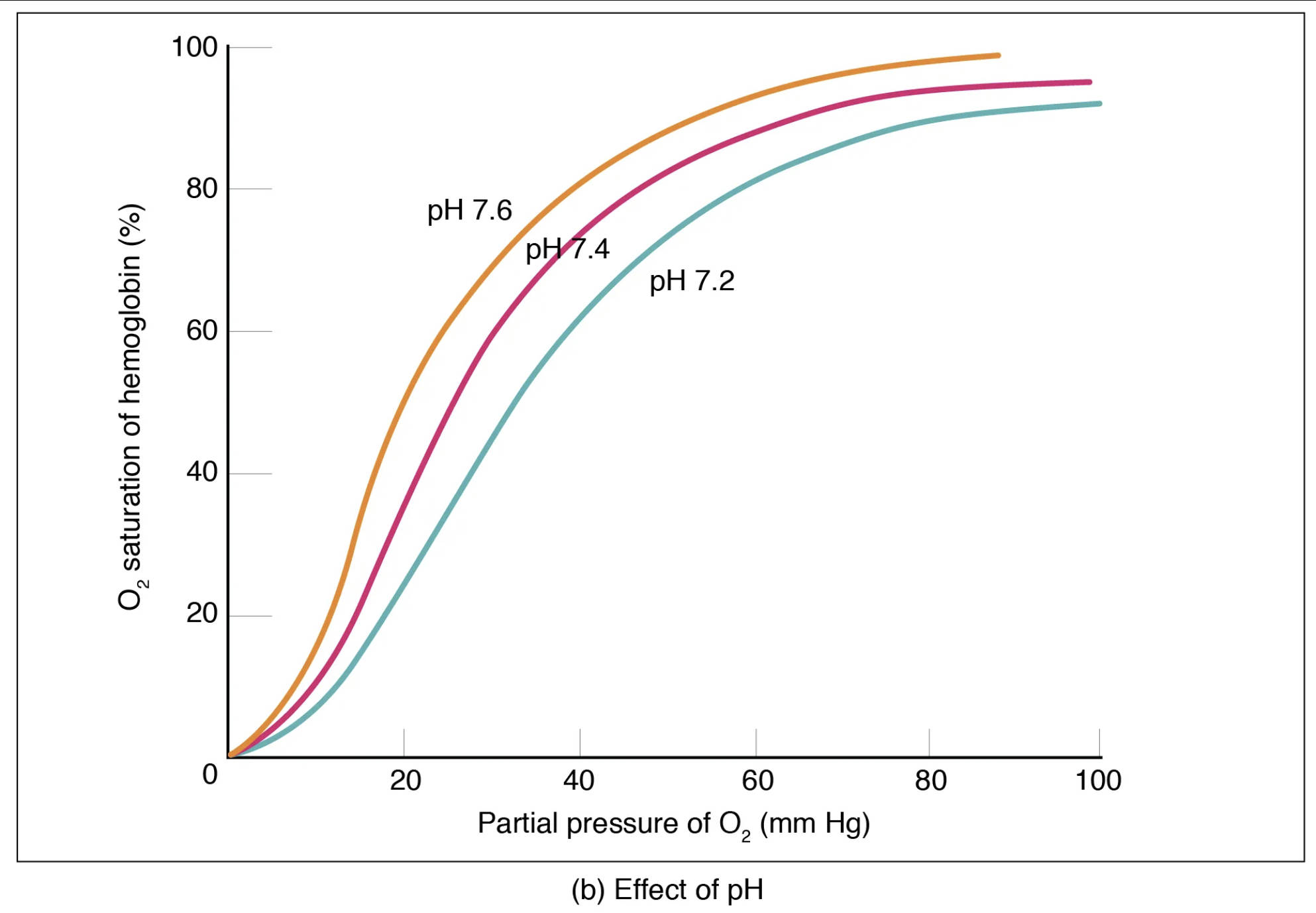
The oxygen-hemoglobin dissociation curve illustrates how the percentage of hemoglobin saturated with oxygen changes with varying partial pressures of oxygen (PO2). However, this curve is not static; it shifts in response to changes in the microenvironment, particularly pH. This phenomenon, where a decrease in blood pH (increased acidity) reduces hemoglobin’s affinity for oxygen, thereby promoting oxygen release to tissues, is known as the Bohr effect. Conversely, an increase in blood pH (decreased acidity) enhances hemoglobin’s affinity for oxygen.
O2 saturation of hemoglobin (%): This vertical axis represents the percentage of hemoglobin molecules that are bound to oxygen. A higher percentage indicates more oxygen being carried.
Partial pressure of O2 (mm Hg): This horizontal axis denotes the partial pressure of oxygen, the driving force for oxygen binding to or dissociating from hemoglobin.
pH 7.6: This curve, shifted to the left, represents a more alkaline (less acidic) blood pH. At this pH, hemoglobin has an increased affinity for oxygen, meaning it binds oxygen more tightly and releases less of it to the tissues for a given PO2. This condition might be seen in the lungs, promoting oxygen uptake.
pH 7.4: This middle curve represents the normal physiological blood pH. At this baseline, oxygen loading and unloading occur at standard rates, reflecting the body’s typical oxygen transport efficiency.
pH 7.2: This curve, shifted to the right, represents a more acidic blood pH. At this pH, hemoglobin has a decreased affinity for oxygen, leading it to release more oxygen to the tissues for a given PO2. This is crucial in metabolically active tissues, which produce more CO2 and H+, thus lowering the pH.
How pH Optimizes Oxygen Delivery
The Bohr effect is a brilliant physiological adaptation that ensures oxygen is delivered precisely where it’s needed most. Metabolically active tissues, such as exercising muscles, produce significant amounts of carbon dioxide (CO2). When CO2 enters the bloodstream, it reacts with water to form carbonic acid, which then dissociates into hydrogen ions (H+) and bicarbonate ions. The increase in H+ ions lowers the local blood pH, making it more acidic.
As the blood becomes more acidic (e.g., pH 7.2), the oxygen-hemoglobin dissociation curve shifts to the right. This rightward shift means that for any given partial pressure of oxygen, hemoglobin is less saturated with oxygen, indicating that it has released more oxygen to the surrounding tissues. This is highly advantageous because active tissues are precisely the areas that require more oxygen for continued metabolic processes. The increased acidity acts as a signal, prompting hemoglobin to relinquish its oxygen cargo more readily.
Conversely, in the lungs, where carbon dioxide is exhaled, the PCO2 decreases, and the blood pH tends to be slightly more alkaline (e.g., pH 7.6). This higher pH causes the oxygen-hemoglobin dissociation curve to shift to the left, increasing hemoglobin’s affinity for oxygen. This enhanced affinity ensures that hemoglobin efficiently picks up oxygen from the alveoli, maximizing oxygen loading before the blood is pumped to the systemic circulation.
Clinical Relevance of the Bohr Effect
The Bohr effect has significant clinical implications. In conditions like metabolic acidosis (e.g., in diabetic ketoacidosis) or respiratory acidosis (e.g., due to hypoventilation), where blood pH is abnormally low, the rightward shift of the curve ensures that tissues receive adequate oxygen despite the systemic imbalance. However, severe or prolonged acidosis can critically impair oxygen binding in the lungs, compromising overall oxygenation. Conversely, in conditions like respiratory alkalosis (e.g., hyperventilation), the leftward shift means oxygen is held more tightly by hemoglobin, potentially reducing its release to tissues even if PO2 is normal, leading to tissue hypoxia. Understanding these pH-induced shifts is crucial for managing patients with acid-base imbalances and ensuring optimal oxygen delivery, especially in critical care settings.
Conclusion
The Bohr effect, illustrating the profound influence of blood pH on the oxygen-hemoglobin dissociation curve, is a testament to the elegant regulatory mechanisms within the human body. This adaptive phenomenon ensures that oxygen is precisely delivered to metabolically active tissues and efficiently loaded in the lungs, responding dynamically to the body’s ever-changing demands. A comprehensive understanding of how pH modulates hemoglobin’s affinity for oxygen is indispensable for medical professionals in diagnosing and managing a wide array of physiological and pathological conditions that impact oxygen transport and overall cellular function.

