Explore the intricate process of external respiration, where oxygen enters the bloodstream and carbon dioxide is released in the lungs. This article details the diffusion across the respiratory membrane and the critical roles of hemoglobin and carbonic anhydrase in facilitating vital gas exchange.
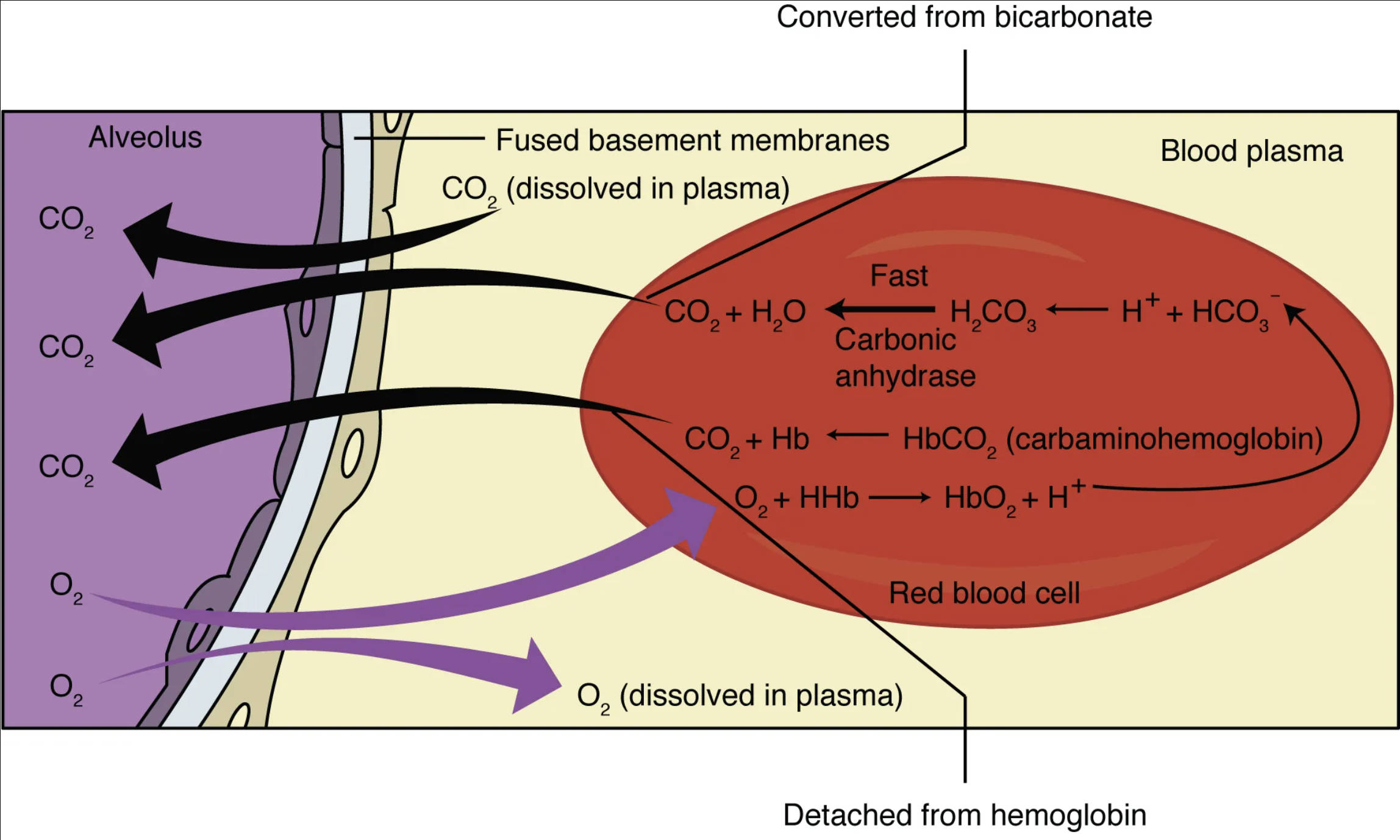
Alveolus: The alveolus is a tiny, air-filled sac in the lungs, where gas exchange primarily occurs. Its extremely thin walls are in close contact with capillaries, forming the respiratory membrane for efficient diffusion.
Fused basement membranes: These membranes represent the extremely thin, combined layers between the alveolar epithelium and the capillary endothelium. Their fusion minimizes the diffusion distance for gases, enhancing the efficiency of oxygen and carbon dioxide exchange.
CO2 (dissolved in plasma): Carbon dioxide can dissolve directly into the blood plasma. While a small percentage, this dissolved CO2 contributes to its partial pressure gradient, driving its diffusion into the alveoli.
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3- (Carbonic anhydrase): This chemical reaction illustrates the rapid conversion of carbon dioxide and water into carbonic acid, which then dissociates into hydrogen ions and bicarbonate ions. The enzyme carbonic anhydrase, found within red blood cells, dramatically speeds up this reversible reaction.
Converted from bicarbonate: This label indicates that a significant portion of carbon dioxide transported in the blood returns to its gaseous form from bicarbonate ions within the red blood cell. Bicarbonate is the primary way CO2 is transported in the blood from tissues to the lungs.
CO2 + Hb ⇌ HbCO2 (carbaminohemoglobin): This reaction shows carbon dioxide binding to hemoglobin (Hb) to form carbaminohemoglobin. This is another method of CO2 transport, though less prevalent than bicarbonate formation.
O2 + HHb ⇌ HbO2 + H+: This equation depicts the binding of oxygen (O2) to deoxyhemoglobin (HHb, hemoglobin that has released its oxygen and is carrying hydrogen ions). This forms oxyhemoglobin (HbO2) and releases hydrogen ions, a process known as the Haldane effect, which facilitates CO2 release from the blood.
Red blood cell: These biconcave cells are the primary transporters of oxygen and, to a lesser extent, carbon dioxide in the blood. Red blood cells contain hemoglobin and the enzyme carbonic anhydrase, both crucial for gas exchange.
Detached from hemoglobin: This indicates that oxygen, after being transported by hemoglobin in the red blood cell, detaches from it to diffuse into the blood plasma and subsequently into tissues. This release is crucial for delivering oxygen where it’s needed.
O2 (dissolved in plasma): A small fraction of oxygen is transported dissolved directly in the blood plasma. While most oxygen is bound to hemoglobin, this dissolved portion contributes to the partial pressure of oxygen in the blood, driving its diffusion.
The Vital Exchange: How Your Lungs Breathe Life into Your Blood
External respiration is a critical physiological process that occurs in the lungs, facilitating the essential exchange of gases between the atmosphere and the blood. This intricate mechanism ensures that the body receives a continuous supply of oxygen, vital for cellular metabolism, while efficiently expelling carbon dioxide, a waste product. The process hinges on the principles of diffusion, driven by partial pressure gradients across the specialized respiratory membrane within the alveoli.
Understanding external respiration is fundamental to comprehending overall respiratory health and the mechanisms by which oxygen is delivered to every cell and carbon dioxide is removed. This complex interplay involves not only the physical movement of gases but also sophisticated chemical reactions occurring within the red blood cells, primarily involving hemoglobin and the enzyme carbonic anhydrase.
Key aspects of external respiration include:
- Diffusion of oxygen from the alveoli into the blood.
- Diffusion of carbon dioxide from the blood into the alveoli.
- The critical role of the respiratory membrane.
This process is a testament to the efficiency and precision of the human body’s design for sustaining life.
The Alveolar-Capillary Dance: Oxygen Uptake
At the heart of external respiration lies the respiratory membrane, an incredibly thin barrier formed by the fused basement membranes of the alveolar epithelium and the capillary endothelium. This delicate structure provides an optimal surface for the rapid diffusion of gases.
Oxygen’s Journey into the Bloodstream
When we inhale, oxygen-rich air fills the alveoli. The partial pressure of oxygen (PO2) in the alveoli is significantly higher than the PO2 in the deoxygenated blood arriving from the pulmonary artery. This steep partial pressure gradient drives oxygen molecules from the alveoli, across the respiratory membrane, and into the blood plasma. A small amount of oxygen dissolves directly into the plasma, but the vast majority then rapidly enters the red blood cells.
Inside the red blood cells, oxygen quickly binds to hemoglobin (Hb), a protein specialized for oxygen transport. This binding forms oxyhemoglobin (HbO2) through a reversible reaction (O2 + HHb ⇌ HbO2 + H+). The binding of oxygen to hemoglobin is influenced by several factors, including pH (the Bohr effect) and temperature, ensuring efficient loading of oxygen in the lungs. This process is highly efficient, allowing the blood to become nearly saturated with oxygen as it passes through the pulmonary capillaries.
Carbon Dioxide Release: From Blood to Air
Simultaneously with oxygen uptake, carbon dioxide, a metabolic waste product, moves from the blood into the alveoli to be exhaled. The partial pressure of carbon dioxide (PCO2) is much higher in the deoxygenated blood arriving at the lungs than in the alveolar air, creating a powerful gradient that drives its diffusion.
Carbon Dioxide Transport and Release Mechanisms
Carbon dioxide is transported in the blood in three main forms:
- Dissolved in Plasma: A small percentage of CO2 (about 7-10%) is transported directly dissolved in the blood plasma. This dissolved CO2 contributes to the PCO2 gradient, facilitating its movement.
- Bound to Hemoglobin (Carbaminohemoglobin): Approximately 20-30% of CO2 binds to the amino groups of hemoglobin, forming carbaminohemoglobin (HbCO2) (CO2 + Hb ⇌ HbCO2). This binding is reversible, and in the oxygen-rich environment of the lungs, CO2 detaches from hemoglobin. The binding of oxygen to hemoglobin (Haldane effect) further enhances the release of CO2 and H+ from hemoglobin, creating a favorable environment for CO2 to leave the blood.
- As Bicarbonate Ions: The most significant portion of CO2 (about 70%) is transported as bicarbonate ions (HCO3-). Within the red blood cells, CO2 rapidly combines with water (H2O) to form carbonic acid (H2CO3), a reaction catalyzed by the enzyme carbonic anhydrase (CO2 + H2O ⇌ H2CO3). Carbonic acid then quickly dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). In the lungs, this process reverses: bicarbonate ions re-enter the red blood cells, combine with hydrogen ions to reform carbonic acid, which is then converted back into CO2 and H2O by carbonic anhydrase. The newly formed CO2 then diffuses out of the red blood cells, into the plasma, across the respiratory membrane, and into the alveoli for exhalation.
Conclusion
External respiration is a testament to the sophisticated design of the human body, showcasing the remarkable efficiency of gas exchange at the alveolar-capillary membrane. The coordinated diffusion of oxygen into the bloodstream and carbon dioxide out of it, facilitated by partial pressure gradients and the critical roles of hemoglobin and carbonic anhydrase within red blood cells, is essential for sustaining life. Understanding these intricate processes is fundamental to appreciating respiratory health and diagnosing conditions that impair this vital exchange.

