The kidneys play a pivotal role in maintaining the body’s acid-base balance, and a crucial aspect of this function is the conservation of bicarbonate. While tubular cells are not directly permeable to bicarbonate, an ingenious mechanism ensures its effective reabsorption back into the bloodstream. This process, primarily occurring in the proximal tubule, is essential for preventing the loss of this vital buffer and maintaining physiological pH. Understanding the steps involved in bicarbonate conservation is fundamental to grasping renal physiology and its impact on systemic acid-base regulation.
Dissecting the Bicarbonate Conservation Pathway
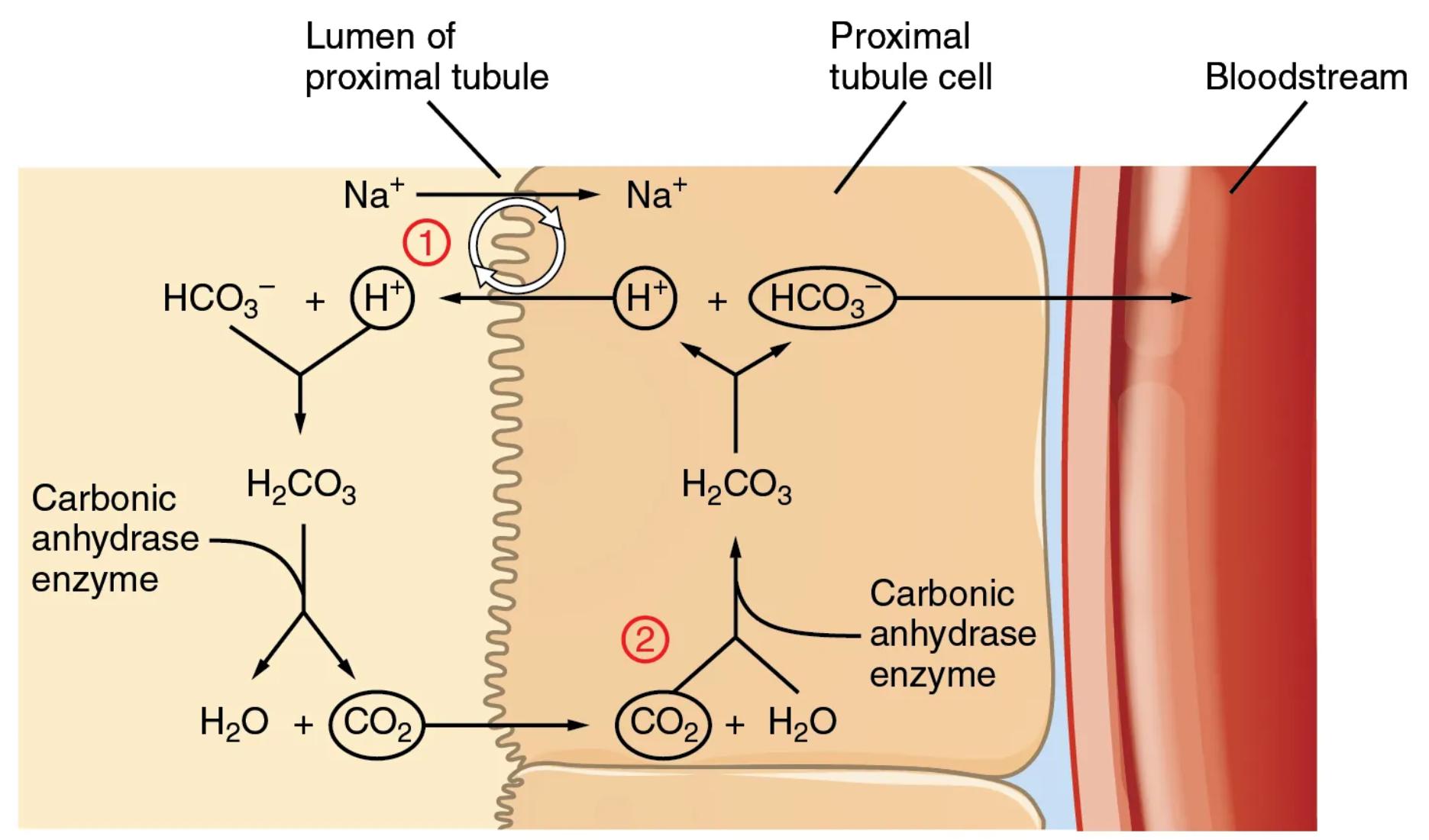
Lumen of proximal tubule: This refers to the inner space of the proximal tubule, which is the initial segment of the renal tubule extending from Bowman’s capsule. It is here that the glomerular filtrate, containing various solutes including bicarbonate (HCO3-), is first processed after leaving the glomerulus. The composition of the filtrate in the lumen will be modified significantly as it travels through the tubule.
Proximal tubule cell: This is an epithelial cell lining the wall of the proximal tubule. These cells are highly specialized with a brush border facing the lumen, increasing their surface area for reabsorption and secretion. They are instrumental in the active transport of ions and water, playing a key role in retrieving essential substances like bicarbonate from the filtrate.
Bloodstream: This represents the peritubular capillaries that surround the renal tubules. After bicarbonate is conserved and effectively “reabsorbed” by the proximal tubule cells, it is then transported into these capillaries to return to the systemic circulation. This ensures that bicarbonate, a critical buffer, remains within the body’s fluid compartments to maintain acid-base homeostasis.
Na+ (Lumen to Proximal tubule cell): Sodium ions (Na+) are actively transported from the lumen of the proximal tubule into the proximal tubule cell. This transport is often coupled with the movement of other ions, creating an electrochemical gradient that drives many reabsorptive processes in the kidney.
HCO3- (Lumen): Bicarbonate ions (HCO3-) are present in the filtrate within the lumen of the proximal tubule. These ions are a major component of the body’s buffer system and their conservation is vital for maintaining blood pH.
H+ (Lumen): Hydrogen ions (H+) are secreted from the proximal tubule cell into the lumen. This secretion is often coupled with sodium reabsorption, facilitated by specific transporters on the apical membrane of the tubular cells.
H2CO3 (Lumen): Carbonic acid (H2CO3) is formed in the lumen when the secreted hydrogen ions (H+) combine with bicarbonate ions (HCO3-) present in the filtrate. This reaction is catalyzed by the enzyme carbonic anhydrase located on the apical membrane of the proximal tubule cells.
Carbonic anhydrase enzyme (Lumen): This enzyme, located on the brush border of the proximal tubule cells, catalyzes the rapid conversion of carbonic acid (H2CO3) into water (H2O) and carbon dioxide (CO2) within the lumen. Its presence is critical for the initial breakdown of carbonic acid, as the tubule cells are not directly permeable to H2CO3.
H2O (Lumen): Water molecules are one of the products formed from the breakdown of carbonic acid in the lumen, facilitated by carbonic anhydrase. This water can then be reabsorbed by the tubular cells, contributing to water balance.
CO2 (Lumen): Carbon dioxide molecules are the other product formed from the breakdown of carbonic acid in the lumen. Unlike bicarbonate, CO2 is highly lipid-soluble and can readily diffuse across the apical membrane into the proximal tubule cell. This permeability is key to its indirect reabsorption.
Na+ (Proximal tubule cell to Lumen): This arrow indicates the reabsorption of sodium ions from the lumen into the proximal tubule cell, often via the Na+/H+ antiporter (NHE3). This particular transporter is crucial for secreting H+ into the lumen while simultaneously reabsorbing Na+.
H+ (Proximal tubule cell): Hydrogen ions (H+) are generated within the proximal tubule cell through the dissociation of intracellular carbonic acid. These H+ ions are then secreted into the lumen, playing a critical role in the bicarbonate conservation process.
HCO3- (Proximal tubule cell): Bicarbonate ions (HCO3-) are also generated within the proximal tubule cell from the dissociation of intracellular carbonic acid. These intracellular HCO3- ions are destined for transport into the bloodstream.
H2CO3 (Proximal tubule cell): Carbonic acid (H2CO3) is formed inside the proximal tubule cell when CO2, which has diffused in from the lumen, combines with intracellular water. This reaction is catalyzed by intracellular carbonic anhydrase.
Carbonic anhydrase enzyme (Proximal tubule cell): This enzyme, located within the cytoplasm of the proximal tubule cell, catalyzes the rapid conversion of CO2 and H2O into H2CO3, and then its dissociation into H+ and HCO3-. Its activity is essential for regenerating bicarbonate for transport into the blood.
CO2 (Proximal tubule cell): Carbon dioxide (CO2) diffuses from the lumen into the proximal tubule cell. Once inside, it is rapidly converted into carbonic acid, initiating the intracellular phase of bicarbonate regeneration.
H2O (Proximal tubule cell): Water (H2O) is utilized within the proximal tubule cell to combine with CO2, forming carbonic acid. This intracellular water is readily available for this reaction.
HCO3- (Proximal tubule cell to Bloodstream): Bicarbonate ions (HCO3-), generated inside the proximal tubule cell, are transported across the basolateral membrane into the bloodstream. This transport is typically facilitated by cotransporters, such as the Na+/HCO3- cotransporter, ensuring bicarbonate’s return to the systemic circulation.
1 (Step 1 of bicarbonate conservation): This label indicates the initial phase where hydrogen ions (H+) are secreted into the lumen, where they combine with filtered bicarbonate (HCO3-) to form carbonic acid (H2CO3). This reaction, catalyzed by luminal carbonic anhydrase, subsequently breaks down H2CO3 into CO2 and H2O. This step is crucial because bicarbonate itself cannot directly cross the tubular cell membrane.
2 (Step 2 of bicarbonate conservation): This label indicates the subsequent phase where the CO2, which readily diffused into the proximal tubule cell, combines with intracellular water to re-form carbonic acid (H2CO3). This reaction is catalyzed by intracellular carbonic anhydrase. The H2CO3 then dissociates into H+ and HCO3-, with the newly formed bicarbonate being transported into the bloodstream. The H+ is then available for secretion into the lumen again, effectively completing a cycle where filtered bicarbonate is “conserved” indirectly.
The diagram “Conservation of Bicarbonate in the Kidney” intricately maps out a vital process occurring primarily in the proximal tubule, demonstrating how the kidneys maintain acid-base homeostasis. Bicarbonate (HCO3-) is an indispensable buffer in the blood, neutralizing excess acids and preventing dangerous shifts in pH. Despite its critical role, tubular cells are not directly permeable to bicarbonate, necessitating an indirect mechanism for its recovery from the glomerular filtrate.
This process essentially involves the “disassembly” of bicarbonate in the tubular lumen and its “reconstruction” within the tubular cells before being returned to the bloodstream. The entire mechanism is highly dependent on the enzyme carbonic anhydrase, found both on the luminal membrane and within the cytoplasm of the proximal tubule cells. The continuous conservation of bicarbonate ensures that this crucial buffer is not lost in the urine, thereby supporting the body’s capacity to manage acid loads.
The steps can be summarized as follows:
- Hydrogen ions (H+) are secreted from the proximal tubule cell into the lumen, where they combine with filtered bicarbonate (HCO3-) to form carbonic acid (H2CO3).
- Luminal carbonic anhydrase rapidly converts H2CO3 into carbon dioxide (CO2) and water (H2O).
- The lipid-soluble CO2 diffuses readily from the lumen into the proximal tubule cell.
- Inside the cell, intracellular carbonic anhydrase catalyzes the reaction of CO2 with H2O to re-form H2CO3.
- H2CO3 then dissociates into H+ and a “new” bicarbonate (HCO3-).
- This newly formed bicarbonate is transported across the basolateral membrane into the bloodstream, while the H+ is available for re-secretion into the lumen, perpetuating the cycle.
This elegant system highlights the kidney’s sophisticated ability to reclaim essential substances, even when direct transport is not possible. The efficiency of this process in the proximal tubule is remarkable, as approximately 80-90% of filtered bicarbonate is conserved here, with the remaining portion handled in later segments of the nephron.
Renal Acid-Base Regulation: The Conservation of Bicarbonate
The kidneys are fundamental organs in the regulation of the body’s acid-base balance, working in concert with the respiratory system to maintain a stable blood pH. A cornerstone of this renal function is the meticulous conservation of bicarbonate (HCO3-), a primary extracellular buffer. Unlike many other filtered substances, bicarbonate cannot directly cross the membranes of renal tubular cells. Instead, the kidney has developed an intricate and highly efficient indirect mechanism to reclaim this vital ion, predominantly in the proximal tubule. This process is crucial for preventing the loss of bicarbonate in the urine and thus averting metabolic acidosis.
The mechanism begins in the lumen of the proximal tubule, where hydrogen ions (H+), generated within the tubular cell, are secreted into the tubular fluid. This secretion is often facilitated by a Na+/H+ antiporter, which simultaneously reabsorbs sodium (Na+) back into the cell. Once in the lumen, these secreted H+ ions readily combine with the filtered bicarbonate (HCO3-) to form carbonic acid (H2CO3). This reaction is swiftly catalyzed by carbonic anhydrase, an enzyme strategically located on the brush border (apical membrane) of the proximal tubule cells. The carbonic anhydrase then breaks down the H2CO3 into carbon dioxide (CO2) and water (H2O).
The resulting CO2 is a lipid-soluble molecule, allowing it to easily diffuse across the apical membrane and into the cytoplasm of the proximal tubule cell. Once inside the cell, another carbonic anhydrase enzyme, this time intracellular, catalyzes the reverse reaction: CO2 combines with intracellular water to re-form carbonic acid (H2CO3). This H2CO3 then dissociates into a “new” hydrogen ion (H+) and a “new” bicarbonate ion (HCO3-). This newly generated bicarbonate is then transported across the basolateral membrane of the tubular cell and into the peritubular capillaries, effectively returning it to the systemic bloodstream. The H+ ions generated intracellularly are then recycled, being secreted back into the lumen to participate in the next round of bicarbonate conversion. This cyclical process ensures the efficient reclamation of virtually all filtered bicarbonate, safeguarding the body’s buffer stores.
The clinical significance of this process is profound. Impairment in renal bicarbonate conservation can lead to various forms of metabolic acidosis, where the body struggles to neutralize accumulating acids. Conditions like renal tubular acidosis (RTA) directly involve defects in these tubular mechanisms, leading to excessive urinary loss of bicarbonate and chronic acidemia. Therefore, understanding the detailed steps of bicarbonate conservation in the proximal tubule is not just an academic exercise but a critical insight into the pathophysiology of numerous acid-base disorders and the fundamental role of the kidneys in maintaining overall physiological equilibrium.
Conclusion
The detailed diagram illustrating the conservation of bicarbonate in the kidney’s proximal tubule reveals an elegant and essential physiological process. Despite the impermeability of tubular cells to bicarbonate, the kidney expertly utilizes enzymatic catalysis and specific transporters to ensure that this crucial buffer is reclaimed from the filtrate and returned to the bloodstream. This intricate mechanism, driven by the coordinated action of hydrogen ion secretion and carbonic anhydrase activity both in the lumen and intracellularly, is fundamental for maintaining the body’s acid-base balance and preventing metabolic acidosis. A comprehensive understanding of these steps is vital for appreciating renal function and its profound impact on systemic physiology.

