The intricate balance of pH within the human body is vital for maintaining physiological functions. This diagram elucidates the critical role of the respiratory system in regulating blood pH, demonstrating the compensatory mechanisms that restore acid/base homeostasis when it is disturbed. Understanding these pathways is fundamental to comprehending the body’s adaptive responses to metabolic challenges.
Understanding the pH Regulation Pathway
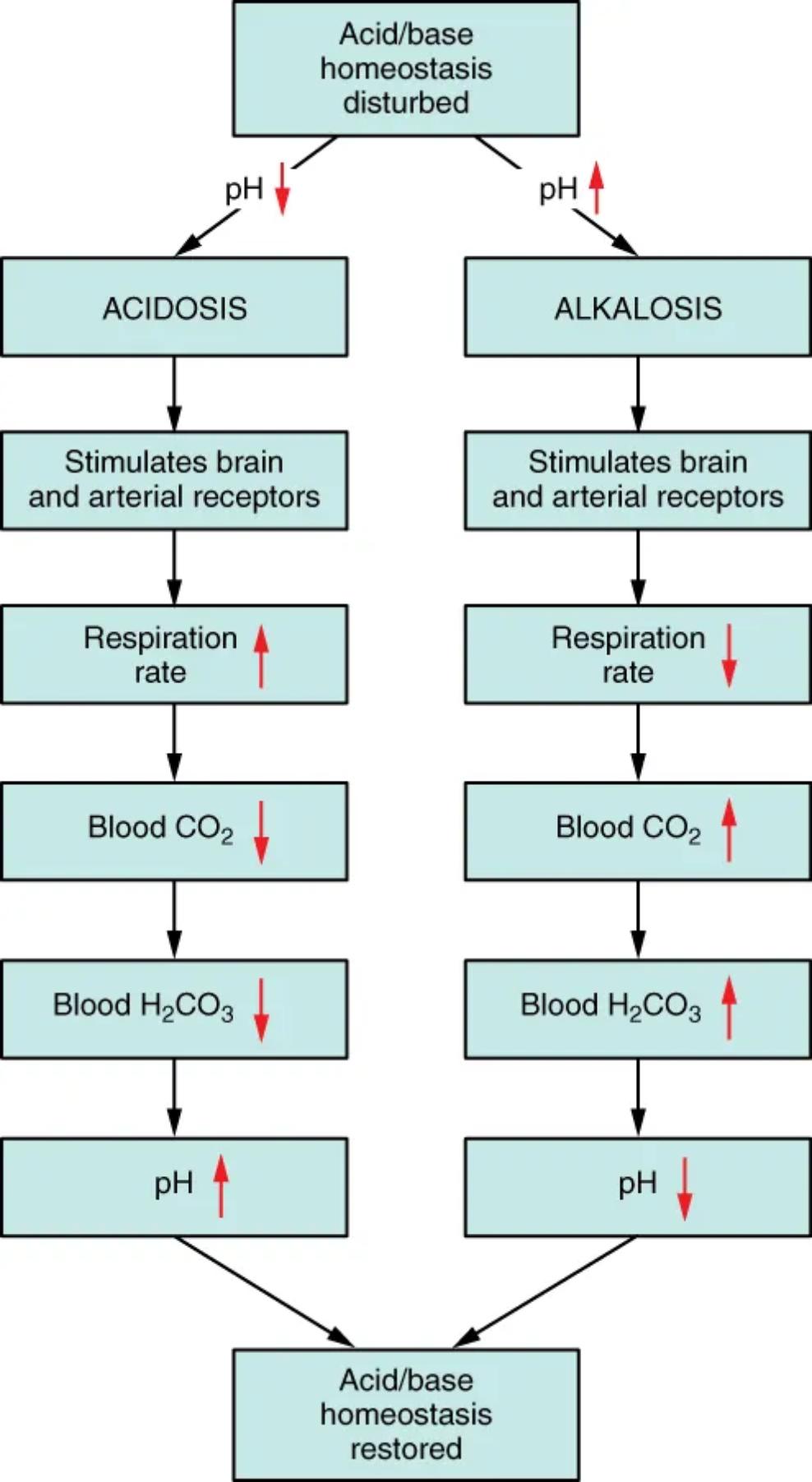
Acid/base homeostasis disturbed: This initial state signifies a deviation from the body’s normal blood pH range, which is tightly maintained between 7.35 and 7.45. Such disturbances can arise from various metabolic or respiratory causes, leading to either an excess of acid or a deficit of acid. This disruption triggers a series of physiological responses aimed at correcting the imbalance and returning the pH to its optimal level.
pH down (Acidosis): A decrease in blood pH below 7.35 indicates a state of acidosis. This condition signifies an increase in hydrogen ion concentration, making the blood more acidic. The body initiates specific compensatory mechanisms to counteract this acidity.
pH up (Alkalosis): An increase in blood pH above 7.45 signifies a state of alkalosis. This condition indicates a decrease in hydrogen ion concentration, making the blood more alkaline or basic. Similar to acidosis, the body activates distinct regulatory processes to correct this alkalinity.
ACIDOSIS: This refers to the physiological condition where the blood pH is abnormally low. It can be caused by an accumulation of metabolic acids or an impaired ability to eliminate carbon dioxide, leading to a build-up of carbonic acid in the blood.
ALKALOSIS: This refers to the physiological condition where the blood pH is abnormally high. It can be caused by excessive loss of metabolic acids or an over-elimination of carbon dioxide, leading to a deficit of carbonic acid in the blood.
Stimulates brain and arterial receptors (Acidosis pathway): In response to acidosis, specialized chemoreceptors located in the brainstem (central chemoreceptors) and in the carotid and aortic bodies (peripheral chemoreceptors) become activated. These receptors are highly sensitive to changes in blood pH and carbon dioxide levels. Their stimulation sends signals to the respiratory control center.
Stimulates brain and arterial receptors (Alkalosis pathway): Similarly, in response to alkalosis, these same chemoreceptors are stimulated, though in a different manner that signals a need to decrease respiratory drive. They detect the low carbon dioxide levels and increased pH, relaying this information to the respiratory center in the brain.
Respiration rate up (Acidosis pathway): The stimulation of chemoreceptors in acidosis leads to a significant increase in the respiration rate. This compensatory hyperventilation is the body’s immediate attempt to expel more carbon dioxide, thereby reducing the amount of carbonic acid in the blood and increasing pH.
Respiration rate down (Alkalosis pathway): In alkalosis, the stimulation of chemoreceptors results in a decrease in the respiration rate. This hypoventilation aims to retain more carbon dioxide in the blood. By holding onto CO2, the body increases carbonic acid levels, which helps to lower the pH.
Blood CO2 down (Acidosis pathway): The elevated respiration rate during acidosis effectively lowers the concentration of carbon dioxide in the blood. This reduction in blood CO2 is a direct consequence of increased exhalation, shifting the bicarbonate buffer system equilibrium.
Blood CO2 up (Alkalosis pathway): Conversely, the reduced respiration rate during alkalosis leads to an increase in blood carbon dioxide levels. By retaining CO2, the body seeks to restore the balance of the carbonic acid-bicarbonate buffer system.
Blood H2CO3 down (Acidosis pathway): As blood CO2 decreases, the equilibrium of the carbonic acid-bicarbonate buffer system shifts, leading to a reduction in carbonic acid (H2CO3) levels in the blood. This is because H2CO3 is formed when CO2 combines with water.
Blood H2CO3 up (Alkalosis pathway): With increased blood CO2 levels during alkalosis, more carbonic acid (H2CO3) is formed. This contributes to increasing the acid component in the blood, which is necessary to counteract the alkaline state.
pH up (Acidosis pathway): The decrease in blood H2CO3 directly results in an increase in blood pH, moving it back towards the physiological normal range. This demonstrates the respiratory system’s effectiveness in compensating for acidic conditions.
pH down (Alkalosis pathway): The increase in blood H2CO3 leads to a decrease in blood pH, bringing it closer to the normal physiological range. This illustrates the respiratory system’s compensatory action against alkaline conditions.
Acid/base homeostasis restored: This final state indicates that the compensatory respiratory mechanisms have successfully corrected the initial pH disturbance. The blood pH has returned to its normal range, signifying the restoration of acid/base balance within the body.
The diagram provides a clear visual representation of how the respiratory system acts as a rapid and effective regulator of blood pH. When a disturbance occurs, leading to either acidosis (low pH) or alkalosis (high pH), the body’s chemoreceptors detect these changes.
- In acidosis, the increased acidity stimulates chemoreceptors, prompting an increase in respiratory rate. This accelerated breathing leads to greater expulsion of carbon dioxide (CO2) from the blood. Since CO2 combines with water to form carbonic acid (H2CO3), reducing CO2 effectively reduces H2CO3, thereby raising the pH back towards normal.
- Conversely, in alkalosis, the elevated pH triggers a decrease in respiratory rate. This slower breathing conserves CO2 within the blood, which then leads to an increase in H2CO3. The higher concentration of carbonic acid helps to lower the pH, restoring the acid/base balance.
This delicate interplay underscores the respiratory system’s crucial role in maintaining the narrow pH range necessary for enzymatic activity, protein structure, and overall cellular function. Without such precise regulation, even minor deviations in blood pH can have profound and detrimental effects on physiological processes. The respiratory system’s immediate response acts as the first line of defense, often working in conjunction with renal mechanisms for longer-term pH control.
The Respiratory System’s Role in pH Regulation
The human body’s internal environment is a marvel of delicate balance, with blood pH being one of the most tightly controlled parameters. Maintaining a blood pH between 7.35 and 7.45 is essential for the proper functioning of enzymes, proteins, and biochemical reactions vital for life. Deviations from this narrow range, known as acidosis or alkalosis, can lead to severe health consequences. The respiratory system plays a pivotal and immediate role in buffering these changes, serving as a rapid-response mechanism to restore acid/base homeostasis.
The core of respiratory pH regulation lies in the reversible reaction between carbon dioxide (CO2) and water (H2O) to form carbonic acid (H2CO3), which then dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-). This relationship is governed by the carbonic acid-bicarbonate buffer system. The lungs control the expulsion or retention of CO2, directly influencing the amount of carbonic acid in the blood.
When the body experiences acidosis, meaning blood pH falls below 7.35, chemoreceptors in the brainstem and arterial system detect the increase in H+ ions and CO2. This triggers a compensatory response: the respiratory center in the brain increases the rate and depth of breathing, a process known as hyperventilation. By breathing faster and deeper, more CO2 is exhaled from the lungs. This reduction in blood CO2 shifts the chemical equilibrium, decreasing the concentration of carbonic acid and, consequently, hydrogen ions. The net effect is an increase in blood pH, moving it back towards the normal range.
Conversely, during alkalosis, when blood pH rises above 7.45, the chemoreceptors sense a decrease in H+ ions and CO2. In response, the respiratory center slows down the breathing rate (hypoventilation). This allows more CO2 to be retained in the blood. The increased CO2 then combines with water to form more carbonic acid, leading to an increase in hydrogen ion concentration. This rise in H+ ions helps to lower the blood pH, thereby restoring the acid/base balance. This elegant feedback loop highlights the respiratory system’s indispensable role in maintaining systemic pH, often working in concert with the renal system for more comprehensive and long-term regulation.
Conclusion
The respiratory system’s ability to swiftly adjust blood pH through the regulation of carbon dioxide levels is a cornerstone of physiological homeostasis. This diagram meticulously illustrates the direct causal links between pH disturbances, chemoreceptor activation, respiratory rate adjustments, and the subsequent changes in blood CO2 and carbonic acid, ultimately leading to the restoration of acid/base balance. A thorough understanding of these mechanisms is critical for appreciating the body’s intrinsic capacity to maintain its internal environment against myriad challenges, ensuring optimal conditions for cellular and systemic function.

