The human body is a remarkable composition of chemical elements, each playing a unique role in maintaining life and function. This image outlines the Oxygen, Carbon, Hydrogen, Nitrogen, Calcium, Phosphorus, Potassium, Sulfur, Sodium, Chlorine, Magnesium, and Iron, listed from most abundant to least abundant, providing insight into their proportional presence. Exploring these elements reveals the foundation of human physiology and their critical contributions to health.
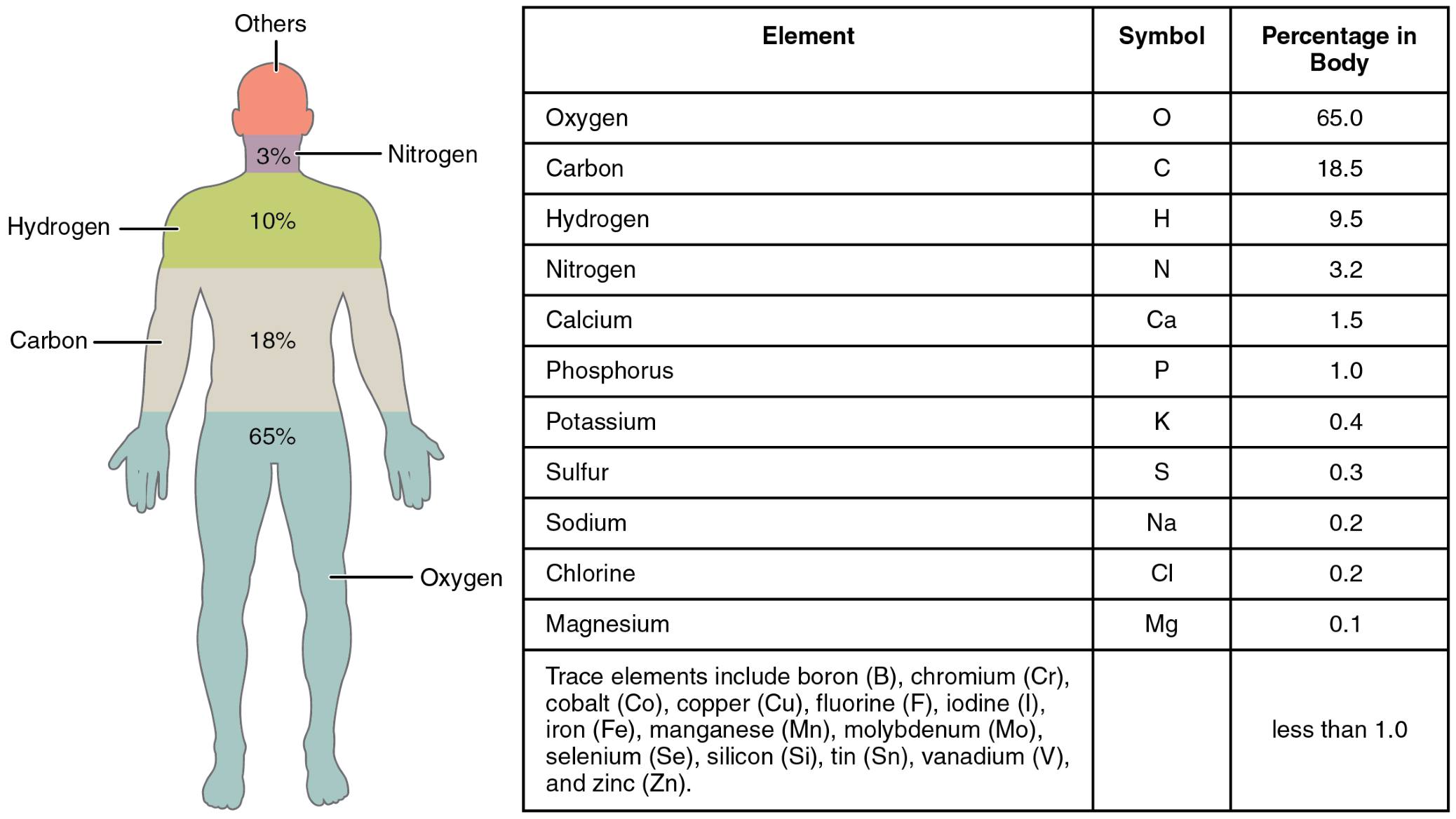
Label Introductions:
- Oxygen: Oxygen is the most abundant element, making up about 65% of the body, primarily in water and organic compounds. It is essential for cellular respiration, enabling energy production in mitochondria.
- Carbon: Carbon constitutes around 18% of the body, forming the backbone of organic molecules like proteins and carbohydrates. It supports the structural integrity of tissues and organs.
- Hydrogen: Hydrogen accounts for approximately 10% of the body, mostly within water molecules. It plays a key role in maintaining pH balance and facilitating chemical reactions.
- Nitrogen: Nitrogen comprises about 3% of the body, found in amino acids and DNA. It is vital for protein synthesis and genetic material formation.
- Calcium: Calcium makes up about 1.5% of the body, concentrated in bones and teeth. It also regulates muscle contraction and nerve signaling as an ion in the bloodstream.
- Phosphorus: Phosphorus, around 1% of the body, is a component of bones, DNA, and ATP. It supports energy transfer and genetic stability within cells.
- Potassium: Potassium, present at about 0.4%, is crucial for nerve function and muscle activity. It maintains fluid balance and supports heart rhythm as an electrolyte.
- Sulfur: Sulfur, approximately 0.3%, is found in amino acids like cysteine and methionine. It contributes to protein structure and detoxification processes.
- Sodium: Sodium, around 0.2%, is an electrolyte that regulates fluid balance and nerve impulses. It works with potassium to maintain cellular homeostasis.
- Chlorine: Chlorine, also about 0.2%, exists as chloride ions, aiding digestion through hydrochloric acid in the stomach. It helps maintain osmotic pressure and acid-base balance.
- Magnesium: Magnesium, around 0.05%, is involved in over 300 enzymatic reactions, including energy production. It supports bone health and muscle relaxation.
- Iron: Iron, less than 0.01%, is critical for hemoglobin, enabling oxygen transport in red blood cells. It also plays a role in energy metabolism and immune function.
Overview of Human Body Elements
The human body is a complex blend of elements, with oxygen, carbon, and hydrogen forming the majority of its composition. These elements combine to create water, proteins, and fats, which are fundamental to life processes. Their distribution and interactions underpin the body’s structural and functional integrity.
- Form the basis of water, the body’s primary constituent.
- Build organic compounds essential for growth and repair.
- Support metabolic processes like respiration and digestion.
- Influence the body’s ability to respond to environmental changes.
Major Elements: Oxygen, Carbon, and Hydrogen
Oxygen, carbon, and hydrogen dominate the body’s elemental makeup, each exceeding 10% of total mass. Oxygen is integral to cellular respiration, while carbon forms the framework of biomolecules, and hydrogen stabilizes water and pH. Together, they create the environment for life-sustaining reactions.
- Oxygen binds with glucose to produce ATP in mitochondria.
- Carbon’s versatility allows for diverse molecular structures.
- Hydrogen ions regulate acidity in bodily fluids.
- These elements are replenished through diet and respiration.
Trace Elements: Calcium and Phosphorus
Calcium and phosphorus are vital trace elements, primarily found in bones and teeth. Calcium strengthens skeletal structure and aids in blood clotting, while phosphorus is a key component of ATP for energy storage. Their balance is crucial for musculoskeletal and metabolic health.
- Calcium ions trigger muscle contractions and neurotransmitter release.
- Phosphorus is part of the phosphate backbone in DNA.
- Deficiency in calcium can lead to osteoporosis.
- Phosphorus supports kidney function in phosphate regulation.
Electrolytes: Potassium, Sodium, and Chlorine
Potassium, sodium, and chlorine act as electrolytes, regulating fluid and nerve function. Potassium maintains heart rhythm, sodium controls blood pressure, and chlorine supports gastric acid production. These elements ensure proper cellular communication and hydration.
- Potassium gradients drive nerve impulse transmission.
- Sodium is reabsorbed in the kidneys to retain water.
- Chlorine aids in killing pathogens in the stomach.
- Imbalances can cause conditions like hypokalemia.
Minor Elements: Sulfur, Magnesium, and Iron
Sulfur, magnesium, and iron are present in smaller amounts but are equally important. Sulfur stabilizes protein structures, magnesium activates enzymes, and iron enables oxygen transport via hemoglobin. Their roles extend to detoxification, energy, and oxygen delivery.
- Sulfur bonds strengthen hair and nail keratin.
- Magnesium is a cofactor in synthesizing T3 and T4 in the thyroid.
- Iron deficiency leads to anemia, reducing oxygen capacity.
- These elements are critical for long-term health maintenance.
Clinical Relevance of Body Elements
The balance of these elements is vital for diagnosing and treating health issues. For instance, low iron levels can indicate anemia, while excess sodium might contribute to hypertension. Monitoring these elements through blood tests helps maintain optimal physiological function.
- Calcium levels affect bone density and nerve signaling.
- Phosphorus imbalances can disrupt energy metabolism.
- Potassium monitoring prevents cardiac arrhythmias.
- Iron supplementation corrects nutritional deficiencies.
Conclusion
The elements of the human body, led by oxygen, carbon, and hydrogen, form the foundation of its complex physiology. From the structural support of calcium and phosphorus to the regulatory roles of potassium and iron, each contributes to overall health. Understanding their proportions and functions empowers better health management and medical interventions.

