The evolution of the human heart becomes increasingly defined by day 22, a stage where the embryonic cardiovascular system takes shape with distinct regions like the truncus arteriosus, bulbus cordis, primitive ventricle, and primitive atrium. This image illustrates the primitive heart tube as it begins to segment into these specialized areas, marking a crucial phase in establishing a functional circulatory system that supports the growing embryo.
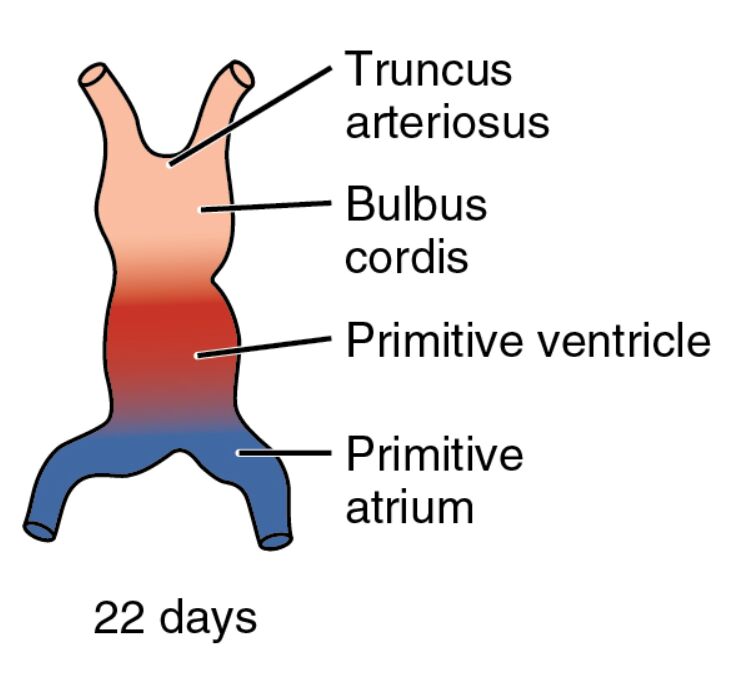
Truncus arteriosus Truncus arteriosus forms the outflow tract of the primitive heart, connecting the heart tube to the developing arterial system. This structure will eventually give rise to the aorta and pulmonary artery through a process of septation, ensuring proper blood distribution.
Bulbus cordis Bulbus cordis is a dilated region proximal to the truncus arteriosus, contributing to the future right ventricle and outflow tracts. This area undergoes significant remodeling to align with the heart’s eventual four-chambered configuration.
Primitive ventricle Primitive ventricle represents the early ventricular chamber, where blood is pumped out of the heart through the bulbus cordis. This region will expand and divide to form the left and right ventricles, critical for systemic and pulmonary circulation.
Primitive atrium Primitive atrium serves as the initial receiving chamber for blood returning via the veins, located at the heart tube’s caudal end. It will later develop into the left and right atria, incorporating contributions from the sinus venosus.
22 days 22 days indicates the gestational age, corresponding to Carnegie stage 10-11, when the heart tube starts beating and segmenting. The embryo, now about 3-4 mm long, exhibits rapid growth and differentiation, with the heart playing a central role in its survival.
The Significance of Heart Tube Segmentation
This stage showcases the heart’s transition from a simple tube to a segmented structure. The division into distinct regions lays the foundation for a complex organ capable of supporting embryonic needs.
- Regional Differentiation: The heart tube’s segmentation into truncus arteriosus, bulbus cordis, primitive ventricle, and primitive atrium is driven by differential gene expression. This process ensures each region develops its specialized function.
- Initiation of Beating: By day 22, the heart tube’s rhythmic contractions begin, propelled by the myocardial layer’s calcium signaling. This beating supports early circulation, delivering nutrients via the yolk sac.
- Morphogenetic Movements: The embryo’s continued folding positions the heart in the thoracic cavity. This alignment facilitates its integration with the developing pharyngeal arches and vascular network.
Anatomical Details of the 22-Day Embryo
The image highlights the heart tube’s elongated S-shape, with color gradients reflecting blood flow direction. These visual cues provide insight into the dynamic changes occurring within the embryo.
The embryo’s size and curvature reflect ongoing morphogenetic processes, protecting the developing heart. The segmentation visible here is a precursor to the heart’s chambered structure.
- Truncus Arteriosus Anatomy: This outflow tract consists of endothelial and myocardial layers, with neural crest cells migrating to aid septation. Its division will form the aorta and pulmonary trunk.
- Bulbus Cordis Structure: Positioned between the ventricle and truncus, this region contains a thick myocardial wall. It contributes to the right ventricle and conus arteriosus during later development.
- Primitive Ventricle Characteristics: The ventricle’s thicker walls indicate its role as the pumping chamber. Its division will create the left and right ventricles, essential for dual circulation.
- Primitive Atrium Features: Located caudally, this chamber receives blood from the sinus venosus and vitelline veins. Its growth involves incorporating the common cardinal veins.
Developmental Milestones at 22 Days
This period marks significant progress in embryonic development, with the heart’s segmentation driving circulatory advancements. The coordination of cardiac and neural growth highlights the intricacy of this phase.
The neural tube continues to close, while somites along the body axis increase in number. These developments parallel the heart’s functional maturation, ensuring holistic embryonic progress.
- Heart Looping Completion: The S-shaped loop fully forms, guided by left-right asymmetry signals like Pitx2. This looping positions the future atria and ventricles correctly.
- Genetic Regulation: Genes such as NKX2-5 and GATA4 direct chamber specification. Mutations can lead to conditions like double outlet right ventricle.
- Vascular Integration: The heart tube connects with the aortic arches and vitelline circulation. This network supports the embryo’s metabolic demands until placental circulation develops.
- Cellular Contributions: Cardiac neural crest cells migrate to the outflow tract, aiding septum formation. This migration is critical for preventing congenital defects.
Physiological Functions of the Segmented Heart
The segmented heart tube begins to perform physiological roles, marking the shift to active circulation. Its beating supports the embryo’s growing oxygen and nutrient requirements.
This early pump operates with low pressure, relying on the yolk sac for oxygenation. The segmentation enhances efficiency, preparing for fetal circulation adaptations.
- Contraction Patterns: The myocardial layer’s coordinated contractions propel blood through the segmented tube. This rhythm strengthens as the heart matures.
- Oxygen Delivery: Primitive erythrocytes, produced in blood islands, carry limited oxygen via hemoglobin. The yolk sac supplements this until the placenta matures.
- Pressure Dynamics: Segmentation creates pressure gradients that drive blood flow. These gradients guide vascular remodeling and arterial development.
- Future Shunts: Structures like the foramen ovale will develop to bypass non-functional lungs. Understanding these adaptations aids in fetal health assessments.
Clinical and Research Perspectives
The 22-day embryo offers valuable insights for medical and scientific fields. The heart’s segmentation is a critical window for studying congenital anomalies.
Advanced imaging and stem cell research enhance our understanding of this stage. These tools hold promise for improving diagnostic and therapeutic approaches.
- Congenital Heart Defects: Errors in segmentation can cause tetralogy of Fallot. Early ultrasound detection allows for timely interventions.
- Regenerative Medicine: Stem cells can be differentiated into chamber-specific cardiomyocytes. This approach supports heart repair strategies.
- Comparative Studies: Zebrafish and chick embryos show similar segmentation patterns. These models validate human developmental findings.
- Therapeutic Targets: Modulating VEGF pathways may enhance vascular growth in defects. Gene editing explores correcting cardiac gene mutations.
In conclusion, this image of the 22-day embryo illustrates the heart’s segmentation into key regions, a pivotal step in cardiovascular development. This early structuring not only sustains embryonic life but also provides a foundation for understanding and addressing congenital heart conditions.

