The human heart begins its intricate formation early in embryonic life, with significant milestones achieved by the eighth week of gestation. At this stage, the heart undergoes partitioning, transforming from a simple tubular structure into a four-chambered organ essential for efficient blood circulation in the fetus. This process involves the development of septa that divide the atria and ventricles, along with the formation of valves that regulate blood flow. Understanding this phase provides insights into congenital heart defects that may arise if partitioning is disrupted. The image illustrates a cross-sectional view of the embryonic heart at 8 weeks, highlighting key anatomical features that support fetal circulation.
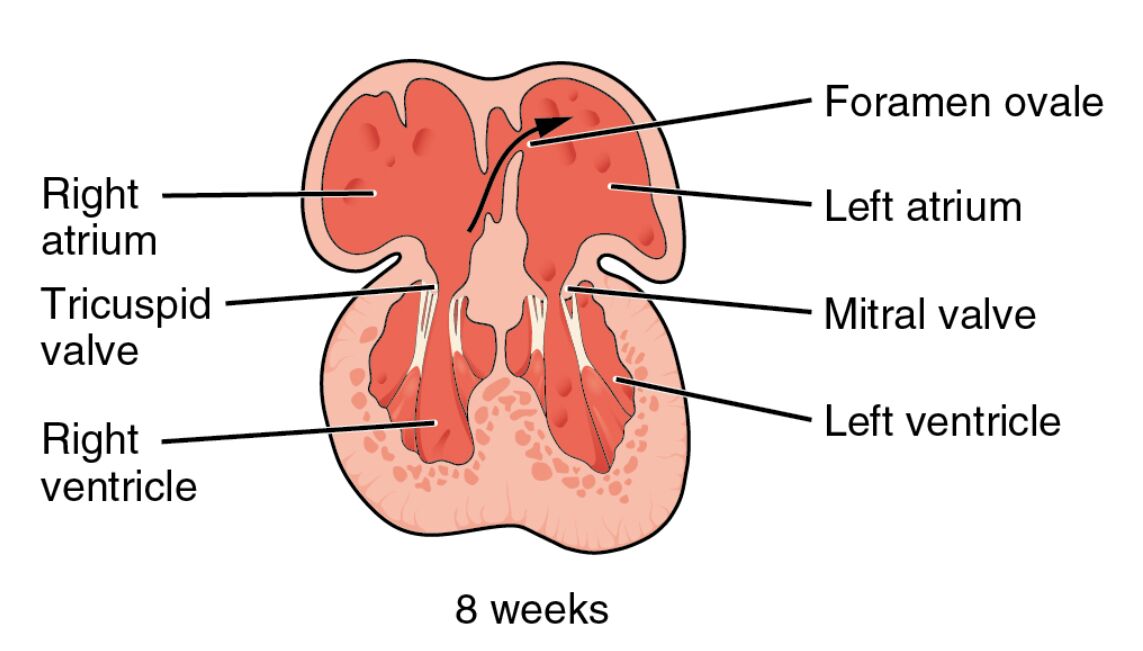
Right atrium The right atrium is one of the upper chambers of the heart, receiving deoxygenated blood from the body via the superior and inferior vena cava. In the embryonic stage, it plays a crucial role in directing blood towards the right ventricle through the tricuspid valve.
Foramen ovale The foramen ovale is an opening between the right and left atria that allows oxygenated blood from the placenta to bypass the pulmonary circulation. This shunt is vital for fetal oxygenation since the lungs are not yet functional, and it typically closes after birth to prevent mixing of oxygenated and deoxygenated blood.
Left atrium The left atrium receives oxygenated blood from the pulmonary veins, which in the fetus primarily comes from the placenta via the umbilical vein. It then pumps this blood into the left ventricle through the mitral valve, ensuring efficient distribution to the body.
Tricuspid valve The tricuspid valve separates the right atrium from the right ventricle, consisting of three cusps that prevent backflow of blood during ventricular contraction. In embryonic development, its formation is critical for maintaining unidirectional blood flow in the right side of the heart.
Mitral valve The mitral valve, also known as the bicuspid valve, has two cusps and guards the opening between the left atrium and left ventricle. It ensures that oxygenated blood flows forward into the systemic circulation without regurgitation.
Right ventricle The right ventricle is the lower chamber on the right side, responsible for pumping deoxygenated blood towards the pulmonary trunk. During fetal life, much of this blood is shunted away from the lungs via the ductus arteriosus to prioritize systemic circulation.
Left ventricle The left ventricle is the thick-walled lower chamber that pumps oxygenated blood into the aorta for distribution throughout the body. In the embryo, its muscular development begins early to handle the demands of fetal growth.
Overview of Heart Partitioning in Embryonic Development
Heart partitioning is a dynamic process that occurs between weeks 4 and 8 of gestation, culminating in the formation of four distinct chambers. This separation is essential for the transition from fetal to postnatal circulation, where oxygenated and deoxygenated blood must be kept apart.
- The process starts with the heart as a straight tube that loops and folds, leading to the formation of primitive atria and ventricles.
- Septa grow to divide the chambers: the interatrial septum separates the atria, while the interventricular septum divides the ventricles.
- Key structures like the foramen ovale and ductus arteriosus (not shown in the image but related) act as temporary shunts to adapt to fetal needs.
- Disruptions in this partitioning can lead to conditions such as atrial septal defects, where the foramen ovale fails to close properly.
- By 8 weeks, the heart is structurally similar to the adult form, though smaller and with fetal adaptations.
- Endocardial cushions contribute to valve formation, including the tricuspid and mitral valves seen in the image.
- Blood flow patterns in the fetus prioritize brain and heart oxygenation, bypassing the lungs.
- Genetic and environmental factors influence this development, with maternal health playing a significant role.
Key Anatomical Features in the 8-Week Embryonic Heart
At 8 weeks, the embryonic heart displays well-defined chambers and valves, as depicted in the cross-sectional illustration. These features ensure efficient circulation tailored to intrauterine life.
- The atria are positioned superiorly, with the right atrium slightly larger to accommodate venous return.
- Ventricles form the bulk of the heart’s mass, with the left ventricle developing thicker walls for systemic pumping.
- Valves like the tricuspid and mitral are derived from mesenchymal tissue, maturing into fibrous structures.
- The foramen ovale appears as a flap-like opening, directing blood from right to left atrium.
- Overall, the heart measures about 5-6 mm in length at this stage, beating at 140-160 times per minute.
- Septal formation involves apoptosis and cell migration, precise processes guided by genes like NKX2-5.
- Imaging techniques such as ultrasound can visualize these structures in vivo during prenatal checkups.
- Comparative anatomy shows similarities across mammals, highlighting evolutionary conservation.
Physiological Implications of Fetal Heart Structure
The physiology of the fetal heart is adapted to a low-oxygen environment, relying on placental exchange rather than pulmonary function. This setup allows for parallel circulation circuits that merge after birth.
- Oxygenated blood from the placenta enters via the umbilical vein, passing through the liver and into the inferior vena cava.
- In the right atrium, this blood is preferentially shunted through the foramen ovale to the left side.
- The right ventricle pumps mostly deoxygenated blood to the pulmonary artery, but the ductus arteriosus diverts it to the aorta.
- Valves prevent mixing and regurgitation, maintaining pressure gradients.
- Cardiac output is high relative to size, supporting rapid embryonic growth.
- Hormonal influences, such as thyroid hormones T3 and T4, regulate heart rate and contractility during development.
- Postnatal changes include closure of shunts and increased pulmonary blood flow.
- Understanding these implications aids in diagnosing fetal arrhythmias or structural anomalies.
Developmental Timeline Leading to 8 Weeks
Heart development initiates around week 3 with the formation of cardiogenic mesoderm. By week 4, the heart tube fuses and begins beating, setting the stage for partitioning.
- Weeks 5-6 see the growth of septa and outflow tract division.
- At week 7, valves start forming from endocardial cushions.
- By 8 weeks, as shown, chambers are partitioned, and the heart is positioned in the thorax.
- Molecular signals like BMP and Wnt pathways orchestrate cell differentiation.
- Nutritional factors, including folic acid, support neural crest cell migration for heart formation.
- Anomalies at this stage may result from teratogens like alcohol or certain medications.
- Echocardiography can detect partitioning completion in fetuses.
- This timeline underscores the vulnerability of early gestation to developmental insults.
Clinical Relevance of Embryonic Heart Partitioning
Knowledge of heart partitioning informs prenatal care and intervention strategies. Early detection of defects can improve outcomes through timely management.
- Congenital heart diseases affect about 1% of live births, often stemming from incomplete partitioning.
- For example, ventricular septal defects occur if the interventricular septum doesn’t fully close.
- Screening ultrasounds at 18-20 weeks build on the 8-week foundation seen here.
- Surgical corrections, like patching septal defects, mimic natural closure mechanisms.
- Research into stem cell therapy aims to regenerate faulty heart tissue based on embryonic models.
- Genetic counseling is advised for families with history of cardiac anomalies.
- Advances in 3D imaging enhance visualization of these early structures.
- Overall, this understanding bridges basic science and clinical practice.
In summary, the partitioning of the heart into four chambers by 8 weeks represents a critical juncture in embryonic development, laying the groundwork for lifelong cardiovascular function. This image captures the elegance of biological engineering, where precise anatomical formations enable survival in the womb and adaptation after birth. Continued research into these processes promises better prevention and treatment of heart-related conditions, emphasizing the importance of maternal and fetal health monitoring.

