Erythrocytes, or red blood cells, are essential for oxygen transport and are continuously produced and recycled to maintain bodily function. This diagram outlines the lifecycle of erythrocytes, from their origin in the bone marrow to their eventual breakdown by macrophages, showcasing the recycling of their components. Gaining insight into this process highlights the body’s remarkable ability to sustain oxygen delivery and manage cellular turnover.
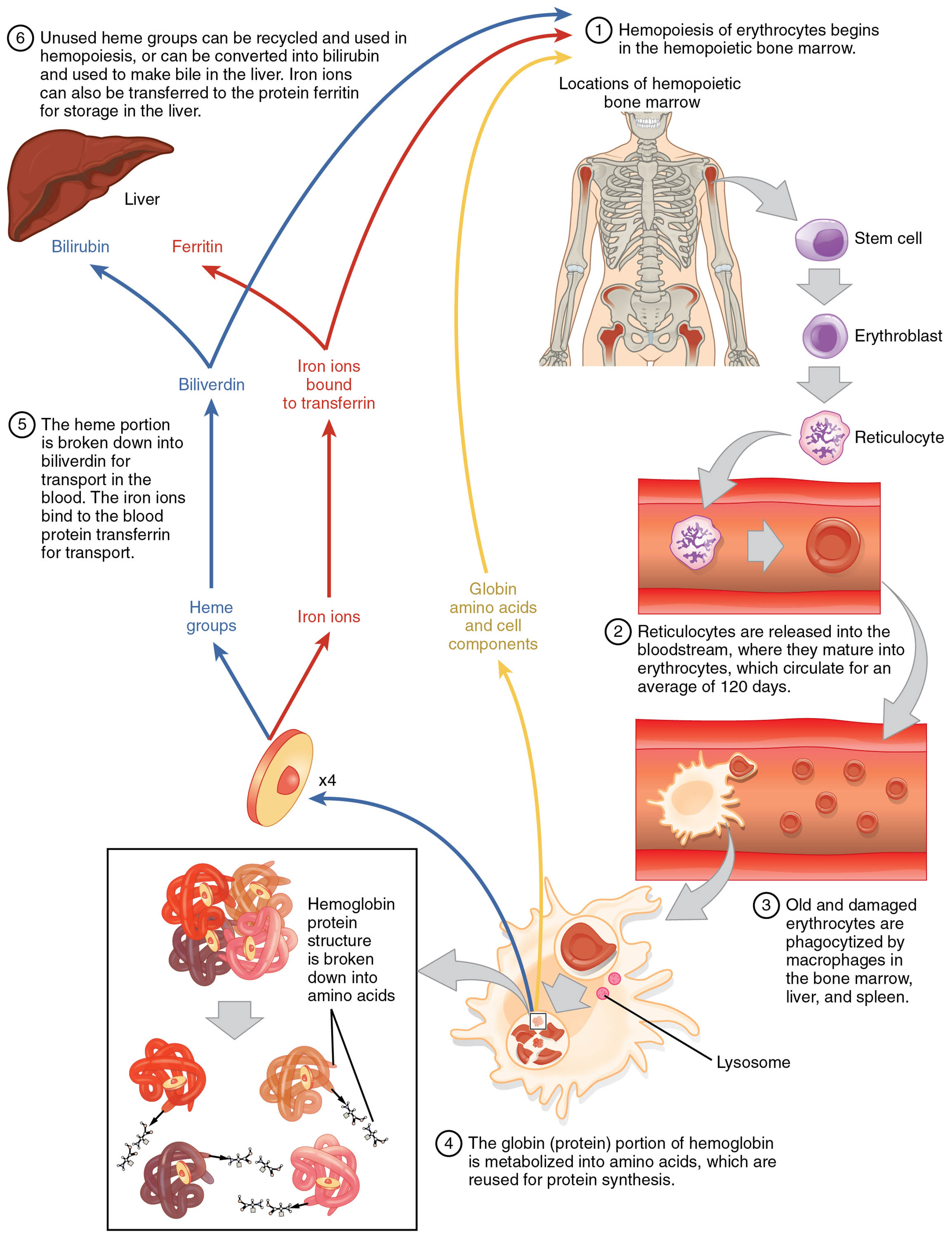
Key Components of the Erythrocyte Lifecycle
The diagram traces the journey and transformation of erythrocytes through distinct stages.
Bone marrow:
The bone marrow serves as the primary site for erythropoiesis, where hematopoietic stem cells differentiate into erythrocytes under the influence of erythropoietin. It provides a nurturing environment with stromal cells and growth factors to support red blood cell production.
Proerythroblast:
The proerythroblast is the earliest recognizable precursor in erythrocyte development, initiating hemoglobin synthesis within the bone marrow. It undergoes several maturation stages, losing its nucleus as it progresses toward functionality.
Basophilic erythroblast:
This stage features a cell with a deep blue cytoplasm due to intense ribosomal activity, marking active protein synthesis. It continues to develop within the bone marrow, preparing for hemoglobin accumulation.
Polychromatophilic erythroblast:
The polychromatophilic erythroblast displays a mix of blue and pink cytoplasm, reflecting a transition in hemoglobin content and ribosomal reduction. It represents a critical intermediate stage before the cell loses its nucleus.
Normoblast:
The normoblast is a late-stage erythroid precursor with a small, dense nucleus and increasing hemoglobin, nearing maturity. It is released into the bloodstream after the nucleus is extruded, becoming a reticulocyte.
Reticulocyte:
The reticulocyte is an immature red blood cell containing residual ribosomal RNA, released into circulation to complete maturation. It typically matures into an erythrocyte within 1-2 days, enhancing oxygen-carrying capacity.
Erythrocyte:
The mature erythrocyte, lacking a nucleus, circulates for about 120 days, transporting oxygen and carbon dioxide via hemoglobin. Its biconcave shape maximizes surface area for gas exchange and flexibility in capillaries.
Aged/damaged erythrocyte:
As erythrocytes age or sustain damage, they become less flexible and are targeted for removal, typically after 120 days in circulation. Their altered membrane properties signal macrophages to initiate clearance.
Macrophage:
Macrophages, primarily located in the spleen and liver, engulf and destroy aged or damaged erythrocytes through phagocytosis. They recycle hemoglobin components, such as iron and amino acids, for reuse in new cell production.
Recycled components:
Recycled components include iron, which is stored as ferritin or transported by transferrin, and amino acids from globin chains. These materials are reutilized in the bone marrow for synthesizing new erythrocytes and other proteins.
The Anatomical and Physiological Role of the Erythrocyte Lifecycle
The erythrocyte lifecycle is a finely tuned process that ensures a constant supply of oxygen-carrying cells, beginning in the bone marrow. Erythropoiesis starts with the proerythroblast, stimulated by erythropoietin from the kidneys in response to hypoxia, with thyroid hormones like T3 and T4 indirectly influencing metabolic oxygen demand. As cells progress through stages like the basophilic erythroblast and polychromatophilic erythroblast, hemoglobin synthesis ramps up, culminating in the normoblast and reticulocyte stages before becoming functional erythrocytes.
Once in circulation, erythrocytes deliver oxygen to tissues and return carbon dioxide, a process aided by the Bohr effect, where low pH enhances oxygen release. After their lifespan, aged/damaged erythrocytes are phagocytosed by macrophages, which break down hemoglobin into recycled components. Iron is stored or reused, while bilirubin from heme is processed by the liver, maintaining iron homeostasis and preventing toxicity.
- Production Phases: Proerythroblasts require vitamin B12 and folate for DNA synthesis; erythropoietin levels rise with anemia.
- Circulation Role: Erythrocytes’ flexibility prevents capillary blockage; their count is regulated by bone marrow activity.
- Recycling Efficiency: Macrophages release iron via ferroportin; bilirubin is conjugated in the liver for excretion.
Physical Characteristics and Clinical Relevance
The physical changes during the erythrocyte lifecycle reflect their functional adaptations, observable through microscopic analysis. Early stages like the proerythroblast are large with prominent nuclei, while mature erythrocytes adopt a biconcave, anucleate shape to optimize gas exchange. The reticulocyte’s residual RNA, visible with stains, indicates recent bone marrow release.
Clinically, this lifecycle is a diagnostic focal point. Low reticulocyte counts suggest bone marrow failure, while high levels indicate hemolysis or blood loss. Disorders like sickle cell disease or thalassemia, affecting hemoglobin structure, shorten erythrocyte lifespan, necessitating transfusions or hydroxyurea. Complete blood counts (CBC) and reticulocyte indices guide treatment, while iron studies assess recycling efficiency.
- Diagnostic Tools: Peripheral smears identify abnormal erythrocyte shapes; serum ferritin measures iron stores.
- Therapeutic Interventions: Erythropoiesis-stimulating agents treat chronic kidney disease; splenectomy addresses hypersplenism.
Conclusion
The erythrocyte lifecycle diagram illustrates the seamless process of red blood cell production, circulation, and recycling, underscoring the body’s ability to maintain oxygen delivery. From the bone marrow’s nurturing environment to the macrophages’ efficient breakdown, each stage reflects a balance of creation and renewal. Understanding this cycle enhances the ability to diagnose and manage conditions affecting erythrocyte function, reinforcing the importance of this vital physiological process.

