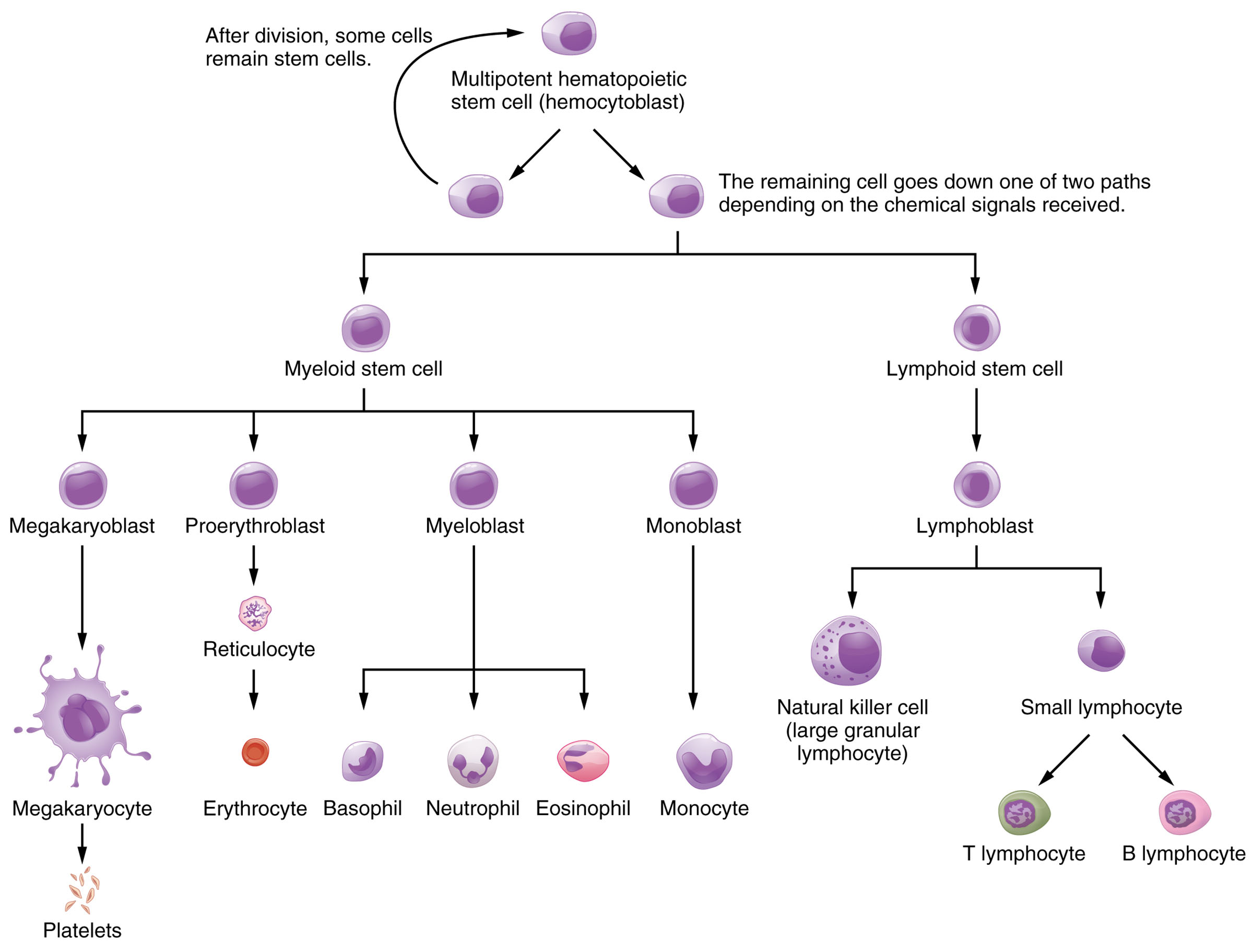
The bone marrow serves as the primary site for hemopoiesis, the dynamic process of producing and differentiating the formed elements of blood, including red blood cells, white blood cells, and platelets. This diagram illustrates the intricate lineage from multipotent hematopoietic stem cells to mature blood cells, highlighting the role of chemical signals in guiding cellular development. Exploring this process offers a deeper understanding of how the body maintains its blood supply and responds to physiological demands.
Key Components of the Hematopoietic System
The diagram outlines the stepwise differentiation of blood cells, starting from a common stem cell.
Multipotent hematopoietic stem cell (hemocytoblast):
This is the foundational cell type capable of self-renewal and differentiation into all blood cell lineages, residing in the bone marrow. After division, some cells remain as stem cells to sustain the population, while others proceed based on chemical cues.
Myeloid stem cell:
The myeloid stem cell is a progenitor that gives rise to a variety of blood cells involved in oxygen transport and innate immunity, branching into multiple lineages. It plays a critical role in responding to demands for red blood cells and granulocytes during stress or infection.
Lymphoid stem cell:
The lymphoid stem cell differentiates into lymphocytes that mediate adaptive immunity, originating from the same hemocytoblast under specific chemical signals. It is essential for long-term immune memory and targeted pathogen defense.
Megakaryoblast:
The megakaryoblast is an early precursor that develops into cells producing platelets, undergoing repeated nuclear divisions without cytokinesis. It eventually forms megakaryocytes, which are vital for blood clotting.
Proerythroblast:
The proerythroblast is the initial stage in red blood cell development, progressing through several maturation steps in the bone marrow. It eventually gives rise to erythrocytes, which are responsible for oxygen transport.
Myeloblast:
The myeloblast is a precursor to granulocytic white blood cells, capable of differentiating into neutrophils, eosinophils, and basophils. It responds to inflammatory signals to increase granulocyte production during infections.
Monoblast:
The monoblast serves as the precursor to monocytes, which later mature into macrophages in tissues, aiding in phagocytosis. It plays a key role in the innate immune response by clearing debris and pathogens.
Lymphoblast:
The lymphoblast is the early stage of lymphocyte development, differentiating into various lymphocyte types under immune system regulation. It is crucial for both innate and adaptive immunity through natural killer and B/T lymphocyte pathways.
Reticulocyte:
The reticulocyte is an immature red blood cell that still contains some ribosomal RNA, released into circulation to mature further. It indicates active erythropoiesis, especially in response to anemia or blood loss.
Megakaryocyte:
The megakaryocyte is a large cell that fragments into platelets, produced from megakaryoblasts in the bone marrow. It ensures adequate platelet numbers for hemostasis and clot formation.
Erythrocyte:
The erythrocyte, or mature red blood cell, lacks a nucleus and is packed with hemoglobin to transport oxygen and carbon dioxide. It has a lifespan of about 120 days, with old cells recycled in the spleen.
Basophil:
The basophil is a granulocyte that releases histamine and heparin during allergic reactions and inflammation, derived from the myeloblast. It constitutes a small percentage of white blood cells but is vital for immediate immune responses.
Neutrophil:
The neutrophil is the most abundant granulocyte, originating from the myeloblast, and is a first responder to bacterial infections. It engulfs pathogens through phagocytosis, often dying in the process to form pus.
Eosinophil:
The eosinophil, derived from the myeloblast, targets parasitic infections and modulates allergic responses by releasing cytotoxic granules. It is less common but critical in specific immune scenarios.
Monocyte:
The monocyte circulates briefly before migrating into tissues as a macrophage, originating from the monoblast, to phagocytize pathogens and present antigens. It supports both innate and adaptive immunity over an extended lifespan.
Natural killer cell (large granular lymphocyte):
The natural killer cell is a lymphoid-derived cell that detects and destroys virus-infected or cancerous cells without prior sensitization. It belongs to the innate immune system, providing rapid defense.
Small lymphocyte:
The small lymphocyte is a mature cell that further differentiates into T and B lymphocytes, derived from the lymphoblast. It is central to adaptive immunity, with a long lifespan to maintain immune memory.
T lymphocyte:
The T lymphocyte, or T cell, develops from the small lymphocyte and mediates cellular immunity by recognizing and destroying infected cells. It includes subsets like helper and cytotoxic T cells, regulated by the thymus.
B lymphocyte:
The B lymphocyte, originating from the small lymphocyte, produces antibodies to neutralize pathogens and supports humoral immunity. It matures in the bone marrow and can differentiate into plasma cells upon activation.
Platelets:
Platelets are cell fragments from megakaryocytes that initiate blood clotting by aggregating at injury sites. They release factors like ADP and serotonin to stabilize clots and promote healing.
The Anatomical and Physiological Role of Hemopoiesis
Hemopoiesis is a finely tuned process that ensures a continuous supply of blood cells, driven by the bone marrow’s microenvironment. The multipotent hematopoietic stem cell (hemocytoblast) resides in the bone marrow’s stromal niche, where it receives growth factors like erythropoietin for red cell production or granulocyte colony-stimulating factor (G-CSF) for white cells.
The myeloid lineage, stemming from the myeloid stem cell, produces erythrocytes that rely on iron and vitamin B12 for hemoglobin synthesis, a process influenced by hypoxia. Lymphoid stem cells give rise to lymphocytes, with T cells maturing in the thymus and B cells in the bone marrow, guided by interleukins and cytokines. Chemical signals, including those from the thyroid gland like T3 and T4, indirectly influence metabolic demands that affect hemopoiesis rates.
- Stem Cell Regulation: Self-renewal is balanced by asymmetric division, ensuring a stem cell pool; differentiation is triggered by bone morphogenetic proteins.
- Erythropoiesis Stages: Proerythroblasts lose nuclei as they mature into reticulocytes; erythrocytes finalize oxygen-binding capacity.
- Immune Cell Development: Lymphoblasts differentiate based on antigen exposure; monocytes adapt into tissue-specific macrophages.
Physical Characteristics and Clinical Significance
The physical transformation of cells during hemopoiesis reflects their specialized functions, observable through microscopic analysis. Erythrocytes adopt a biconcave shape to maximize surface area for gas exchange, while neutrophils develop multilobed nuclei for mobility.
Clinically, this diagram aids in diagnosing disorders by tracing lineage disruptions. For example, low reticulocyte counts suggest bone marrow failure, while elevated myeloblasts may indicate leukemia. Bone marrow biopsies assess these stages, guided by the chart’s lineage map, to tailor treatments like stem cell transplants or growth factor therapy.
- Diagnostic Tools: Flow cytometry identifies cell surface markers on lymphoblasts; hemoglobin electrophoresis detects thalassemia.
- Therapeutic Approaches: G-CSF boosts neutrophil production in neutropenia; erythropoietin treats chronic kidney disease anemia.
Conclusion
The hematopoietic system diagram reveals the remarkable complexity of blood cell production, from the versatile hemocytoblast to specialized cells like erythrocytes and lymphocytes. This process underscores the body’s ability to adapt to oxygen needs, immune challenges, and injury through precise cellular differentiation. Mastery of these pathways enhances the ability to address hematological conditions effectively, highlighting the bone marrow’s critical role in sustaining life.

